Periodic Table Notes Odysseyware Vocabulary the average relative
... The Modern Periodic Table Notice that the elements are arranged in horizontal ____________ and vertical ________________. All the elements of the same ____________ have similar _______________ and ______________ properties. The periods all show a similar _______________, with metallic elements on th ...
... The Modern Periodic Table Notice that the elements are arranged in horizontal ____________ and vertical ________________. All the elements of the same ____________ have similar _______________ and ______________ properties. The periods all show a similar _______________, with metallic elements on th ...
Chemistry Fall Final Review Worksheet Part 1
... b. Students know chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many ...
... b. Students know chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many ...
Periodic Table – an arrangement of the elements in order of their
... Semimetal – an element that does not have metallic properties; found on right side of the periodic table. Valence electron – an electron in the outermost energy level of an atom; for most atoms, it’s available to be gained, lost, or shared in the formation of chemical bonds. Abbreviated electron con ...
... Semimetal – an element that does not have metallic properties; found on right side of the periodic table. Valence electron – an electron in the outermost energy level of an atom; for most atoms, it’s available to be gained, lost, or shared in the formation of chemical bonds. Abbreviated electron con ...
Science Review Sheet: Periodic Table Test Name: ______ Study
... 4. What do valence electrons tell us about an atom? ...
... 4. What do valence electrons tell us about an atom? ...
Organizing Clutter: The Organizing of the Elements of the Universe
... You are going to use your knowledge of atomic structure and Bohr’s model to build the class periodic table. You will work with a partner and each of you will build at least one atom of an element. Each of you will have: At least one element card with information about the element Paper plates – one ...
... You are going to use your knowledge of atomic structure and Bohr’s model to build the class periodic table. You will work with a partner and each of you will build at least one atom of an element. Each of you will have: At least one element card with information about the element Paper plates – one ...
Ways the Periodic Table is Organized
... table is organized. Be sure to give examples as well as the definition: A: Groups (p. 22) B: Periods (p. 22) C: Reactivity (p. 26) ...
... table is organized. Be sure to give examples as well as the definition: A: Groups (p. 22) B: Periods (p. 22) C: Reactivity (p. 26) ...
Revision of Chemistry work
... 2. Name 4 other elements not in the first 20 on the periodic table and give their chemical symbol. 3. Name two elements that have been named after planets. 4. Make a word using element symbols. 5. Which are there most of in the periodic table? Solids, Liquids or Gases. 6. Which are there less of in ...
... 2. Name 4 other elements not in the first 20 on the periodic table and give their chemical symbol. 3. Name two elements that have been named after planets. 4. Make a word using element symbols. 5. Which are there most of in the periodic table? Solids, Liquids or Gases. 6. Which are there less of in ...
Periodic Table
... Flame spectra of Db are green, and those of Df are red. Db is more dense than Df. Df is used to impart a crimson color to fireworks. DbSO4 is often taken internally by patients who undergo X-ray examination of the gastrointestinal tract. GROUP F Elements of group F have four valence electrons. F ...
... Flame spectra of Db are green, and those of Df are red. Db is more dense than Df. Df is used to impart a crimson color to fireworks. DbSO4 is often taken internally by patients who undergo X-ray examination of the gastrointestinal tract. GROUP F Elements of group F have four valence electrons. F ...
Counting Atoms and Balancing Chemical Equations
... Hydrogen is an element. Oxygen is an element. When hydrogen and oxygen bond they make the compound water. When salt and water are combined, a mixture is created. Compounds in mixtures retain their individual properties. ...
... Hydrogen is an element. Oxygen is an element. When hydrogen and oxygen bond they make the compound water. When salt and water are combined, a mixture is created. Compounds in mixtures retain their individual properties. ...
The Periodic Table Notes
... Have seven valence electrons Very good at stealing an electron from other compounds which make them great oxidizers Known as halogens because they react with metals to create salts At room temperature, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid ...
... Have seven valence electrons Very good at stealing an electron from other compounds which make them great oxidizers Known as halogens because they react with metals to create salts At room temperature, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid ...
5.1 Structure of the Periodic Table
... number from the mass number, you can determine the number of neutrons. Mass Number – Atomic Number = Neutrons ...
... number from the mass number, you can determine the number of neutrons. Mass Number – Atomic Number = Neutrons ...
L2: The Atom, Standard Notation and Borh
... Use the periodic table (p.647 in your textbook) to help you. The atomic number is on the top left side of each element symbol on the periodic table and the atomic mass is below each element symbol. For example carbon has atomic number 6 and mass of 12. (Note: The mass is rounded to the nearest whole ...
... Use the periodic table (p.647 in your textbook) to help you. The atomic number is on the top left side of each element symbol on the periodic table and the atomic mass is below each element symbol. For example carbon has atomic number 6 and mass of 12. (Note: The mass is rounded to the nearest whole ...
20 Questions
... 13. Which groups on the Periodic Table are commonly used for things like tools, coins, or jewelry? ...
... 13. Which groups on the Periodic Table are commonly used for things like tools, coins, or jewelry? ...
Chemistry
... Compound – when two or more elements combine chemically and form a new substance. Compounds have three important characteristics (properties): (1) Compounds have a definite composition (2) Compounds can be broken down into simpler substances by chemical means and (3) Compounds can be identified by t ...
... Compound – when two or more elements combine chemically and form a new substance. Compounds have three important characteristics (properties): (1) Compounds have a definite composition (2) Compounds can be broken down into simpler substances by chemical means and (3) Compounds can be identified by t ...
Elements and the Periodic Table
... 14. Looking at the periodic table and based on its atomic number, how many protons does Carbon have. 15 .How are elements arranged in the periodic table? ...
... 14. Looking at the periodic table and based on its atomic number, how many protons does Carbon have. 15 .How are elements arranged in the periodic table? ...
Subatomic Particles
... • Atoms of the same _________ that have the same number of ________ (p+) but different numbers of ________ (n°) are known as _________ of that element. • _________ of an element are represented b adding the number that indicates the ___________ (A) of hat isotope to the ...
... • Atoms of the same _________ that have the same number of ________ (p+) but different numbers of ________ (n°) are known as _________ of that element. • _________ of an element are represented b adding the number that indicates the ___________ (A) of hat isotope to the ...
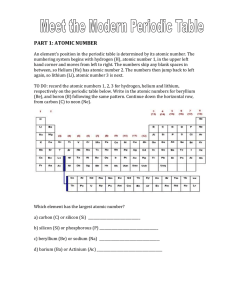
PART 1: ATOMIC NUMBER - hrsbstaff.ednet.ns.ca
... 2. Find all of the gasses in the periodic table and with a black pencil crayon, outline each of them on the above table. 3. In the table below, indicate which gasses are located in each of the periods. Your table will not have elements in every blank space. ...
... 2. Find all of the gasses in the periodic table and with a black pencil crayon, outline each of them on the above table. 3. In the table below, indicate which gasses are located in each of the periods. Your table will not have elements in every blank space. ...
Chapter 8 Study Guide
... Period- (7) rows of the periodic table; elements are NOT similar in same period Most of the elements are classified as metals. Properties of elements change as you go across a period. Alkali Metals I- most reactive metals Alkaline Earth Metals II- not as reactive as Alkali Metals Halogens (VII)- mos ...
... Period- (7) rows of the periodic table; elements are NOT similar in same period Most of the elements are classified as metals. Properties of elements change as you go across a period. Alkali Metals I- most reactive metals Alkaline Earth Metals II- not as reactive as Alkali Metals Halogens (VII)- mos ...
HISTORY OF THE PERIODIC TABLE
... IX 1930 Glenn Seaborg – “plucked out” the heaviest Elements (Actinide series & Lanthanide series) X ROY ALEXANDER – designed a three-dimensional Periodic chart (1994) retains the separate Lanthanide and Actinide series. ...
... IX 1930 Glenn Seaborg – “plucked out” the heaviest Elements (Actinide series & Lanthanide series) X ROY ALEXANDER – designed a three-dimensional Periodic chart (1994) retains the separate Lanthanide and Actinide series. ...
Elements and the Periodic Table
... 13. Looking at the periodic table, what is the atomic mass of Carbon? ...
... 13. Looking at the periodic table, what is the atomic mass of Carbon? ...
Document
... 17. The subatomic particle that plays the greatest role in determining the physical and chemical properties of an element is the a. proton. c. electron. b. neutron. d. photon. 18. Which of the following atoms would you expect to have the largest atomic radius? a. I c. Ca b. K d. Rb 19. From left to ...
... 17. The subatomic particle that plays the greatest role in determining the physical and chemical properties of an element is the a. proton. c. electron. b. neutron. d. photon. 18. Which of the following atoms would you expect to have the largest atomic radius? a. I c. Ca b. K d. Rb 19. From left to ...
Name Periodic Table Assignment Directions: Using your text (pgs
... Periodic Table? What are the characteristics of metals? What are the characteristics of nonmetals? Which elements are considered to be metalloids (semimetals)? What are their properties? Define Atomic Radius. What is its trend as you go across a period? What is its trend as you go down a group? When ...
... Periodic Table? What are the characteristics of metals? What are the characteristics of nonmetals? Which elements are considered to be metalloids (semimetals)? What are their properties? Define Atomic Radius. What is its trend as you go across a period? What is its trend as you go down a group? When ...
Period 3 element
A period 3 element is one of the chemical elements in the third row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when the periodic table skips a row and a chemical behaviour begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. Note that there is a 3d orbital, but it is not filled until Period 4, such giving the period table its characteristic shape of ""two rows at a time"". All of the period 3 elements occur in nature and have at least one stable isotope.