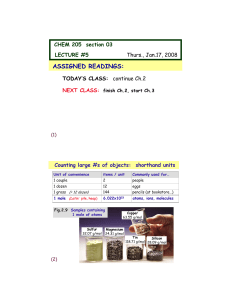
Basics of the Periodic Table
... *Directions: Use the Periodic Table in your textbook to answer the following questions. Read carefully! ...
... *Directions: Use the Periodic Table in your textbook to answer the following questions. Read carefully! ...
Document
... Lanthanides and actinides “rare earth elements” subset of transition metals… Common metals: Fe, Cu, Ni, Zn… In nature: typically found as compounds • In elemental form in nature: Ag, Au, Pt (…quite unreactive) Most react with air (oxidation), but not violently: rusting of Fe • Most elemental t ...
... Lanthanides and actinides “rare earth elements” subset of transition metals… Common metals: Fe, Cu, Ni, Zn… In nature: typically found as compounds • In elemental form in nature: Ag, Au, Pt (…quite unreactive) Most react with air (oxidation), but not violently: rusting of Fe • Most elemental t ...
Section 14.2 - CPO Science
... of one or more elements. • Most metals are used as alloys and not in their pure elemental form. • Yellow brass is an alloy of 72% copper, 24% zinc, 3% lead, and 1% tin. ...
... of one or more elements. • Most metals are used as alloys and not in their pure elemental form. • Yellow brass is an alloy of 72% copper, 24% zinc, 3% lead, and 1% tin. ...
ATOMS ELEMENTS PERIODIC TABLE MOLECULES COMPOUNDS
... • Two types of chemical bonds common in compounds are covalent bonds and ionic bonds. ...
... • Two types of chemical bonds common in compounds are covalent bonds and ionic bonds. ...
CI_Chap_1_Test_A_Study_Guide
... Transition metals like Gold are toward the middle of the periodic table. The most common element in the universe is hydrogen. Atoms of an element always have a certain number of protons. Isotopes of an element differ in the number of neutrons. When an atom loses one or more electrons in becomes an i ...
... Transition metals like Gold are toward the middle of the periodic table. The most common element in the universe is hydrogen. Atoms of an element always have a certain number of protons. Isotopes of an element differ in the number of neutrons. When an atom loses one or more electrons in becomes an i ...
PERIODIC TABLE - WordPress.com
... 3. What is atomic number? 4. What are the atomic numbers and relative atomic masses of Sodium and Chlorine? 5. What are d-block elements commonly known as? 6. Name three metalloids (semi-metals) from the Periodic Table. 7. Which block (s, p, d, f) does iron belong to in the Periodic Table? 8. Which ...
... 3. What is atomic number? 4. What are the atomic numbers and relative atomic masses of Sodium and Chlorine? 5. What are d-block elements commonly known as? 6. Name three metalloids (semi-metals) from the Periodic Table. 7. Which block (s, p, d, f) does iron belong to in the Periodic Table? 8. Which ...
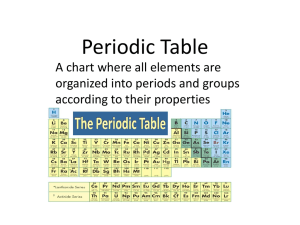
Periodic table
... The exercise below is based on concept mapping. It provides a concept map for the periodic table, with links labelled with numbers. Students are asked to supply sentences to match the links. Below are some suggested sentences that relate to each of the numbers on the diagram. They are not the only s ...
... The exercise below is based on concept mapping. It provides a concept map for the periodic table, with links labelled with numbers. Students are asked to supply sentences to match the links. Below are some suggested sentences that relate to each of the numbers on the diagram. They are not the only s ...
http://www.sps186.org/downloads/attachments/36092/Periodic%20Table%20Worksheet.pdf
... C. Argon __________ ...
... C. Argon __________ ...
Name
... (T) are in group 14. T has more protons than Hi. The element called Yazzer (Y) is a metalloid by position but its properties suggest it is a light metal. The most reactive metal on the planet is Xtalt (X). The most reactive nonmetal is called apstrom (A). The lightest element on the planet is called ...
... (T) are in group 14. T has more protons than Hi. The element called Yazzer (Y) is a metalloid by position but its properties suggest it is a light metal. The most reactive metal on the planet is Xtalt (X). The most reactive nonmetal is called apstrom (A). The lightest element on the planet is called ...
The Atom and how it is organized - Cashmere
... The atoms of all elements are made up of a central nucleus with orbiting electrons. ◦ A nucleus is made up of positively charged PROTONS and neutral NEUTRONS. ◦ ELECTRONS are negatively charged and orbit around the nucleus. ...
... The atoms of all elements are made up of a central nucleus with orbiting electrons. ◦ A nucleus is made up of positively charged PROTONS and neutral NEUTRONS. ◦ ELECTRONS are negatively charged and orbit around the nucleus. ...
File
... found on the right hand side of the periodic table__________________________ make up the majority of the periodic table__________________________ **Use the figure below to answer questions 27-30** 27. Which elements have the same number of ...
... found on the right hand side of the periodic table__________________________ make up the majority of the periodic table__________________________ **Use the figure below to answer questions 27-30** 27. Which elements have the same number of ...
Atoms, elements and compounds
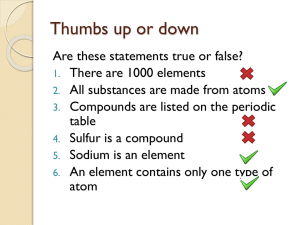
... Are these statements true or false? 1. There are 1000 elements 2. All substances are made from atoms 3. Compounds are listed on the periodic table 4. Sulfur is a compound 5. Sodium is an element 6. An element contains only one type of atom ...
... Are these statements true or false? 1. There are 1000 elements 2. All substances are made from atoms 3. Compounds are listed on the periodic table 4. Sulfur is a compound 5. Sodium is an element 6. An element contains only one type of atom ...
UNIT 3 –TEST REVIEW 1 Atoms of which of the
... Zinc IS IN SAME GROUP AS Cd F gold (Au) G zinc (Zn) H silver (Ag) J copper (Cu) ...
... Zinc IS IN SAME GROUP AS Cd F gold (Au) G zinc (Zn) H silver (Ag) J copper (Cu) ...
Name: Per: _____ Date: ______ Unit 5 Redemption Packet: The
... 1. Mendeleev arranged the elements in his periodic table by increasing _______________ ________________. 2. Why did Mendeleev leave spaces in his periodic table? _________________________________________________________________________ 3. The modern periodic table is arranged by increasing _________ ...
... 1. Mendeleev arranged the elements in his periodic table by increasing _______________ ________________. 2. Why did Mendeleev leave spaces in his periodic table? _________________________________________________________________________ 3. The modern periodic table is arranged by increasing _________ ...
View PDF
... a. atomic number. b. atomic mass. c. mass number. d. atomic mass unit. 5. How is the atomic mass of an element determined? a. Average the atomic masses of all its isotopes. b. Use the atomic mass of the most abundant isotope. c. Take a weighted average of the masses of the isotopes present in nature ...
... a. atomic number. b. atomic mass. c. mass number. d. atomic mass unit. 5. How is the atomic mass of an element determined? a. Average the atomic masses of all its isotopes. b. Use the atomic mass of the most abundant isotope. c. Take a weighted average of the masses of the isotopes present in nature ...
Unit 3 Practice Test
... 2. While exploring the bottom of the ocean floor, you come across a very cool element that you need to identify. After running some laboratory tests, you determine that the element is a gas at room temperature, doesn’t bind with any other elements, and doesn’t conduct electricity. a. Do you think it ...
... 2. While exploring the bottom of the ocean floor, you come across a very cool element that you need to identify. After running some laboratory tests, you determine that the element is a gas at room temperature, doesn’t bind with any other elements, and doesn’t conduct electricity. a. Do you think it ...
The Periodic Table Chemistry – Leaving Cert Quick Notes
... The Periodic Table An element is a substance that cannot be split into simpler substances by chemical means. Humphry Davy isolated potassium and sodium from their hydroxide compounds. Henry Moseley used the concept of atomic number to derive another definition for an element: a substance all of tho ...
... The Periodic Table An element is a substance that cannot be split into simpler substances by chemical means. Humphry Davy isolated potassium and sodium from their hydroxide compounds. Henry Moseley used the concept of atomic number to derive another definition for an element: a substance all of tho ...
Worksheet 1, UNIT THREE
... __________________________________________ than the last one. 3. As you move from left to right across a period on the periodic table the size of an atom will __________________________________________. 4. This happens because as you move across a period each element has one more _______________ in ...
... __________________________________________ than the last one. 3. As you move from left to right across a period on the periodic table the size of an atom will __________________________________________. 4. This happens because as you move across a period each element has one more _______________ in ...
Section 15.2 - CPO Science
... elements Almost all the molecules that make up plants and animals are constructed around carbon. The chemistry of carbon is so important it has its own name, organic chemistry. ...
... elements Almost all the molecules that make up plants and animals are constructed around carbon. The chemistry of carbon is so important it has its own name, organic chemistry. ...
Section 12.4 - CPO Science
... 12.4 Carbon and carbon-like elements Almost all the molecules that make up plants and animals are constructed around carbon. The chemistry of carbon is so important it has its own name, organic chemistry. ...
... 12.4 Carbon and carbon-like elements Almost all the molecules that make up plants and animals are constructed around carbon. The chemistry of carbon is so important it has its own name, organic chemistry. ...
Chemistry 104: Introduction to the Chemistry of Materials
... Grammar and Spelling 2 points Although the paper will be only a few paragraphs long, the information should be reported in complete sentences. Please use double spacing. Content 6 points 1. Identify the element: give its name, chemical symbol, atomic number, period and group. 2. Describe physical pr ...
... Grammar and Spelling 2 points Although the paper will be only a few paragraphs long, the information should be reported in complete sentences. Please use double spacing. Content 6 points 1. Identify the element: give its name, chemical symbol, atomic number, period and group. 2. Describe physical pr ...
Period 3 element
A period 3 element is one of the chemical elements in the third row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when the periodic table skips a row and a chemical behaviour begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. Note that there is a 3d orbital, but it is not filled until Period 4, such giving the period table its characteristic shape of ""two rows at a time"". All of the period 3 elements occur in nature and have at least one stable isotope.