The Periodic Table - Miss Schaefer`s Science Grade 8
... • Mendeleev was the first scientist to notice the relationship between the elements – Arranged his periodic table by atomic mass – Said properties of unknown elements could be predicted by the properties of elements around the missing element ...
... • Mendeleev was the first scientist to notice the relationship between the elements – Arranged his periodic table by atomic mass – Said properties of unknown elements could be predicted by the properties of elements around the missing element ...
trend lab
... Purpose: You will arrange the code elements in groups 1&2 (s block) and 13-18 ( the p block) (the entire d and f block is not present )according to atomic number, symbol, oxidation number, atomic radii, and electronegativity. Materials: Scissors, glue, tables Procedure: 1. Cut out all the blocks of ...
... Purpose: You will arrange the code elements in groups 1&2 (s block) and 13-18 ( the p block) (the entire d and f block is not present )according to atomic number, symbol, oxidation number, atomic radii, and electronegativity. Materials: Scissors, glue, tables Procedure: 1. Cut out all the blocks of ...
Atoms and the
... The complete symbol for an atom or ion consists of the elemental symbol surrounded by subscripts and superscripts. a) The leading superscript (upper left) is the mass number. This is also the number of nucleons; a nucleon is a proton or a neutron. b) The leading subscript (lower left) is the atomic ...
... The complete symbol for an atom or ion consists of the elemental symbol surrounded by subscripts and superscripts. a) The leading superscript (upper left) is the mass number. This is also the number of nucleons; a nucleon is a proton or a neutron. b) The leading subscript (lower left) is the atomic ...
Atoms and the
... The complete symbol for an atom or ion consists of the elemental symbol surrounded by subscripts and superscripts. a) The leading superscript (upper left) is the mass number. This is also the number of nucleons; a nucleon is a proton or a neutron. b) The leading subscript (lower left) is the atomic ...
... The complete symbol for an atom or ion consists of the elemental symbol surrounded by subscripts and superscripts. a) The leading superscript (upper left) is the mass number. This is also the number of nucleons; a nucleon is a proton or a neutron. b) The leading subscript (lower left) is the atomic ...
Chemistry Study Guide - Atomic structure and the Periodic Table 2010
... b. How can you use the periodic table to identify elements in simple compounds. 7. The organization of the periodic table is based on the properties of the elements and reflects the structure of atoms. As a basis for understanding this concept: a. Identify regions of the periodic table that correspo ...
... b. How can you use the periodic table to identify elements in simple compounds. 7. The organization of the periodic table is based on the properties of the elements and reflects the structure of atoms. As a basis for understanding this concept: a. Identify regions of the periodic table that correspo ...
GO 3_3 The Periodic Table
... They react when exposed to air or water As you move down the group, reactivity increases. Lithium ...
... They react when exposed to air or water As you move down the group, reactivity increases. Lithium ...
Year 10 Chemistry Exam June 2012
... c) forms because the ions in a solution repel each other. d) collects at the surface when two solutions are mixed. 14. When solutions of sodium chloride (NaCl) and silver nitrate (AgNO3) are mixed, solid silver chloride (AgCl) forms. Which ions remain dissolved in the solution? a) Na+ and Cl. b) Ag ...
... c) forms because the ions in a solution repel each other. d) collects at the surface when two solutions are mixed. 14. When solutions of sodium chloride (NaCl) and silver nitrate (AgNO3) are mixed, solid silver chloride (AgCl) forms. Which ions remain dissolved in the solution? a) Na+ and Cl. b) Ag ...
WS #10 - Atomic Theory and Periodic Table
... electrons more strongly. This atom has therefore acquired an extra share of negative charge and begins to resemble a negative ion. The other atom correspondingly begins to resemble a positive ion. The extent to which this sharing of an electron pair is unequal is indicated by the percentage ionic ch ...
... electrons more strongly. This atom has therefore acquired an extra share of negative charge and begins to resemble a negative ion. The other atom correspondingly begins to resemble a positive ion. The extent to which this sharing of an electron pair is unequal is indicated by the percentage ionic ch ...
Chapter 6 Studyguide: The Periodic Table
... 36.In going from left to right in any given row in the periodic table, the size of atoms generally _____. 37.Compared to the neutral atom from which it is derived, a negative ion is _____in size. 38.The valence configuration shared by carbon, silicon, and germanium is _____. 39.Transition elements, ...
... 36.In going from left to right in any given row in the periodic table, the size of atoms generally _____. 37.Compared to the neutral atom from which it is derived, a negative ion is _____in size. 38.The valence configuration shared by carbon, silicon, and germanium is _____. 39.Transition elements, ...
In the space provided, write the letter of the term or phrase that best
... b. are very reactive elements. c. are rare elements. d. each have a stable octet. ______ 8. To which group does hydrogen belong? a. Group 1 b. Group 2 ...
... b. are very reactive elements. c. are rare elements. d. each have a stable octet. ______ 8. To which group does hydrogen belong? a. Group 1 b. Group 2 ...
1. In what order did Mendeleev arrange the elements in his periodic
... 2. What family of elements was unknown when Mendeleev created the periodic table? a) noble gases b) alkali metals c) alkaline earth metals d) halogens 3. Mendeleev predicted the existence of which then unknown element? a) zinc b) silicon c) aluminum d) germanium 4. In Mendeleev's periodic table, som ...
... 2. What family of elements was unknown when Mendeleev created the periodic table? a) noble gases b) alkali metals c) alkaline earth metals d) halogens 3. Mendeleev predicted the existence of which then unknown element? a) zinc b) silicon c) aluminum d) germanium 4. In Mendeleev's periodic table, som ...
NAME: Unit 3 Test Review Arsenic (As), Selenium (Se), and
... 6. Which has a higher atomic mass metals or non metals? 7. The valence electrons determine an elements chemical property and they also determine how an element ________ with other elements. 8. What are the three main subatomic particles of an atom? 9. Which group on the periodic table is made of ONL ...
... 6. Which has a higher atomic mass metals or non metals? 7. The valence electrons determine an elements chemical property and they also determine how an element ________ with other elements. 8. What are the three main subatomic particles of an atom? 9. Which group on the periodic table is made of ONL ...
Nomenclature Notes
... Step 1: Look up the names of the elements on the periodic table by using the symbols given in the formula. Step 2: Use prefixes when naming a nonmetal-nonmetal compound. The number following the symbol indicates the prefix to use. *The prefixes used in compounds containing two nonmetals are: ...
... Step 1: Look up the names of the elements on the periodic table by using the symbols given in the formula. Step 2: Use prefixes when naming a nonmetal-nonmetal compound. The number following the symbol indicates the prefix to use. *The prefixes used in compounds containing two nonmetals are: ...
THE PERIODIC TABLE
... 30. Which element in each pair has the higher electronegativity value? a. Na, Mg b. Rb, I c. Cl, Br ...
... 30. Which element in each pair has the higher electronegativity value? a. Na, Mg b. Rb, I c. Cl, Br ...
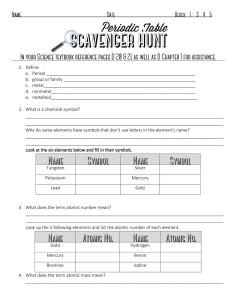
1. Define: a. Period b. group or family
... _________________________________________________________________________________ _________________________________________________________________________________ Why do some elements have symbols that don’t use letters in the element’s name? ________________________________________________________ ...
... _________________________________________________________________________________ _________________________________________________________________________________ Why do some elements have symbols that don’t use letters in the element’s name? ________________________________________________________ ...
File
... THE PERIODIC TABLE TODAY Lesson Objective: Relate patterns in the physical and chemical properties of the elements to their positions in the periodic table. ...
... THE PERIODIC TABLE TODAY Lesson Objective: Relate patterns in the physical and chemical properties of the elements to their positions in the periodic table. ...
THE PERIODIC TABLE TODAY
... THE PERIODIC TABLE TODAY Lesson Objective: Relate patterns in the physical and chemical properties of the elements to their positions in the periodic table. ...
... THE PERIODIC TABLE TODAY Lesson Objective: Relate patterns in the physical and chemical properties of the elements to their positions in the periodic table. ...
Periodic Table Test Chemistry 1 1. What is the horizontal row in the
... 4. What type of element is a good conductor of heat and electric current? 5. What type of element is characterized by the presence of electrons in the d orbital? 6. What is one-half the distance between the nuclei of two atoms when the atoms are joined? 7. What type of ion formed by Group 2A element ...
... 4. What type of element is a good conductor of heat and electric current? 5. What type of element is characterized by the presence of electrons in the d orbital? 6. What is one-half the distance between the nuclei of two atoms when the atoms are joined? 7. What type of ion formed by Group 2A element ...
THE PERIODIC TABLE
... 30. Which element in each pair has the higher electronegativity value? a. Na, Mg b. Rb, I c. Cl, Br ...
... 30. Which element in each pair has the higher electronegativity value? a. Na, Mg b. Rb, I c. Cl, Br ...
3.08_Periodic Table and the Atom
... 22. Elements of Groups 17 are called ________________________________. 23. The most active element in Group 17 is ________________________________. 24. Elements of Groups 18 are called ________________________________. 25. What sublevels are filling across the Transition Metals? ________________ 26. ...
... 22. Elements of Groups 17 are called ________________________________. 23. The most active element in Group 17 is ________________________________. 24. Elements of Groups 18 are called ________________________________. 25. What sublevels are filling across the Transition Metals? ________________ 26. ...
MS Word Printable
... 5. Find potassium. What is the atomic #? ______ atomic mass? ______ # of neutrons? _____ 6. In an electrically neutral carbon atom, how many protons are present? ____ how many electrons? _____ 7. The elements in the far right column, group 8 or VIII, are considered stable in terms of bonding. Name t ...
... 5. Find potassium. What is the atomic #? ______ atomic mass? ______ # of neutrons? _____ 6. In an electrically neutral carbon atom, how many protons are present? ____ how many electrons? _____ 7. The elements in the far right column, group 8 or VIII, are considered stable in terms of bonding. Name t ...
Activity - Periodic Table Scavenger Hunt
... SWBAT use the periodic table to name elements, given their symbols. SWBAT use the periodic table to write the symbols of elements, given their names SWBAT describe the arrangement of the periodic table SWBAT list the characteristics that distinguish metals, nonmetals and metalloids. SWBAT efficientl ...
... SWBAT use the periodic table to name elements, given their symbols. SWBAT use the periodic table to write the symbols of elements, given their names SWBAT describe the arrangement of the periodic table SWBAT list the characteristics that distinguish metals, nonmetals and metalloids. SWBAT efficientl ...
Physical properties
... Periodic Table Vocabulary Identify each word with its definition. Each word can only be used once. ...
... Periodic Table Vocabulary Identify each word with its definition. Each word can only be used once. ...
The Periodic Table
... • Fluorine, Chlorine, Bromine.(Fl, Cl, Br) • Each element has 7 electrons in its outer shell. ...
... • Fluorine, Chlorine, Bromine.(Fl, Cl, Br) • Each element has 7 electrons in its outer shell. ...
Period 3 element
A period 3 element is one of the chemical elements in the third row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when the periodic table skips a row and a chemical behaviour begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. Note that there is a 3d orbital, but it is not filled until Period 4, such giving the period table its characteristic shape of ""two rows at a time"". All of the period 3 elements occur in nature and have at least one stable isotope.