IT`S ATOMIC
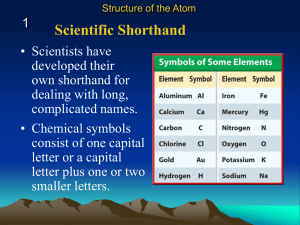
... Scientists all over the world use the same symbols for the elements. Chemical symbols are a shorthand way of writing the chemical names of the elements. Symbols for the elements usually consist of one or two letters. Some man-made elements have three letters in their chemical symbols. If the chemica ...
... Scientists all over the world use the same symbols for the elements. Chemical symbols are a shorthand way of writing the chemical names of the elements. Symbols for the elements usually consist of one or two letters. Some man-made elements have three letters in their chemical symbols. If the chemica ...
Periodicity
... outermost s and nearby d sublevel contain electrons. The inner transition metals: In these metallic elements, the outermost s and nearby f sublevel generally contain electrons. ...
... outermost s and nearby d sublevel contain electrons. The inner transition metals: In these metallic elements, the outermost s and nearby f sublevel generally contain electrons. ...
Periodic Table
... patterns in their properties. Chemists used the properties of elements to sort them into groups Ex. Chlorine, bromine, and iodine have very similar chemical properties. ...
... patterns in their properties. Chemists used the properties of elements to sort them into groups Ex. Chlorine, bromine, and iodine have very similar chemical properties. ...
Notes powerpoint
... Russian chemist, searched for a way to organize the elements. • When he arranged all the elements known at that time in order of increasing atomic masses, he discovered a pattern. ...
... Russian chemist, searched for a way to organize the elements. • When he arranged all the elements known at that time in order of increasing atomic masses, he discovered a pattern. ...
Periodic Table
... patterns in their properties. Chemists used the properties of elements to sort them into groups Ex. Chlorine, bromine, and iodine have very similar chemical properties. ...
... patterns in their properties. Chemists used the properties of elements to sort them into groups Ex. Chlorine, bromine, and iodine have very similar chemical properties. ...
1 Periodic table and atomic structure
... Learning Outcomes Leaving Certificate Chemistry define and explain atomic orbitals describe the shapes of s and p orbitals build up the electronic structure of the first 36 elements derive the electronic configurations of ions of s- and p block elements only describe the arrangement of electrons in ...
... Learning Outcomes Leaving Certificate Chemistry define and explain atomic orbitals describe the shapes of s and p orbitals build up the electronic structure of the first 36 elements derive the electronic configurations of ions of s- and p block elements only describe the arrangement of electrons in ...
1 Periodic table and atomic structure
... Learning Outcomes Leaving Certificate Chemistry define and explain atomic orbitals describe the shapes of s and p orbitals build up the electronic structure of the first 36 elements derive the electronic configurations of ions of s- and p block elements only describe the arrangement of electrons in ...
... Learning Outcomes Leaving Certificate Chemistry define and explain atomic orbitals describe the shapes of s and p orbitals build up the electronic structure of the first 36 elements derive the electronic configurations of ions of s- and p block elements only describe the arrangement of electrons in ...
Notes - RCSD
... same element have the same number of protons 4. Atom: the smallest amount of an element that still has the properties of the element; it is made of protons, neutrons, and electrons 5. Proton: a positively charged, heavy particle in the nucleus of an atom; the number of protons in an atom determines ...
... same element have the same number of protons 4. Atom: the smallest amount of an element that still has the properties of the element; it is made of protons, neutrons, and electrons 5. Proton: a positively charged, heavy particle in the nucleus of an atom; the number of protons in an atom determines ...
The Organization of the Elements
... Another problem Mendeleev encountered was that sometimes the next heaviest element in his list did not fit the properties of the next available place on the table. He would skip places on the table, leaving holes, in order to put the element in a group with elements with similar properties. For exam ...
... Another problem Mendeleev encountered was that sometimes the next heaviest element in his list did not fit the properties of the next available place on the table. He would skip places on the table, leaving holes, in order to put the element in a group with elements with similar properties. For exam ...
HS standard 4 2017
... C) atomic number. D) valence number. Explanation: Although there are many different organizations of the periodic table, the one that determines how elements will actually be placed is determined by the number of protons, or the atomic number 10) Radon is the heaviest naturally radioactive _________ ...
... C) atomic number. D) valence number. Explanation: Although there are many different organizations of the periodic table, the one that determines how elements will actually be placed is determined by the number of protons, or the atomic number 10) Radon is the heaviest naturally radioactive _________ ...
Periods and Blocks of the Periodic Table
... • Mendeleev noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals. • Repeating patterns are referred to as periodic. • Mendeleev created a table in which elements with similar properties were g ...
... • Mendeleev noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals. • Repeating patterns are referred to as periodic. • Mendeleev created a table in which elements with similar properties were g ...
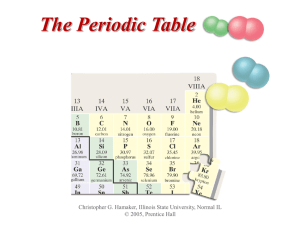
The Periodic Table
... • In 1829, the German chemist J. W. Döbereiner observed that several elements could be classified into groups of three, or triads. • All three elements in a triad showed very similar chemical properties and an orderly trend in physical properties. ...
... • In 1829, the German chemist J. W. Döbereiner observed that several elements could be classified into groups of three, or triads. • All three elements in a triad showed very similar chemical properties and an orderly trend in physical properties. ...
c1l2ch06
... There have been a number of additions to Mendeleev's table since he first proposed it, but the underlying principle that elements could be arranged by properties is still the same. ...
... There have been a number of additions to Mendeleev's table since he first proposed it, but the underlying principle that elements could be arranged by properties is still the same. ...
The Periodic Table
... Adjusting the Periodic Table • About 40 years after Mendeleev’s table, Henry Moseley made an important change to the periodic table: ▫ Organized elements by atomic number instead of atomic mass. ▫ Elements that had not previously fit into the correct column when ordered by atomic mass were fixed. ...
... Adjusting the Periodic Table • About 40 years after Mendeleev’s table, Henry Moseley made an important change to the periodic table: ▫ Organized elements by atomic number instead of atomic mass. ▫ Elements that had not previously fit into the correct column when ordered by atomic mass were fixed. ...
The Periodic Table
... Adjusting the Periodic Table • About 40 years after Mendeleev’s table, Henry Moseley made an important change to the periodic table: ▫ Organized elements by atomic number instead of atomic mass. ▫ Elements that had not previously fit into the correct column when ordered by atomic mass were fix ...
... Adjusting the Periodic Table • About 40 years after Mendeleev’s table, Henry Moseley made an important change to the periodic table: ▫ Organized elements by atomic number instead of atomic mass. ▫ Elements that had not previously fit into the correct column when ordered by atomic mass were fix ...
Periodic Table Funsheet
... 11. Elements of Group 1 are called _____________________________________________________________. 12. Elements of Group 2 are called _____________________________________________________________. 13. Elements of Group 3-12 are called __________________________________________________________. 14. Gr ...
... 11. Elements of Group 1 are called _____________________________________________________________. 12. Elements of Group 2 are called _____________________________________________________________. 13. Elements of Group 3-12 are called __________________________________________________________. 14. Gr ...
Chapter6
... 1. Metals - most of the elements are metals (about 80%). Metals are good conductors of heat and electricity. They have luster or sheen due to their ability to reflect light. All metals are solid at room temperature except for mercury. Most metals are ductile (can be drawn into wire) and malleable (a ...
... 1. Metals - most of the elements are metals (about 80%). Metals are good conductors of heat and electricity. They have luster or sheen due to their ability to reflect light. All metals are solid at room temperature except for mercury. Most metals are ductile (can be drawn into wire) and malleable (a ...
Atomic Radius File - Galena Park ISD Moodle
... Name ______________________________________________ Chemistry Analyzing Trends in Data – Atomic Radius ...
... Name ______________________________________________ Chemistry Analyzing Trends in Data – Atomic Radius ...
Students should be able to describe
... Elements in the same group in the periodic table have the same number of electrons in their outer shell (outer electrons) and this gives them similar chemical properties. Students should be able to: o explain how the position of an element in the periodic table is related to the arrangement of elect ...
... Elements in the same group in the periodic table have the same number of electrons in their outer shell (outer electrons) and this gives them similar chemical properties. Students should be able to: o explain how the position of an element in the periodic table is related to the arrangement of elect ...
PeriodicTrends
... Periodic Table to predict properties of elements. • SC4a. Use the Periodic Table to predict periodic trends including atomic radii, ionic radii, ionization energy, and electronegativity of various elements. ...
... Periodic Table to predict properties of elements. • SC4a. Use the Periodic Table to predict periodic trends including atomic radii, ionic radii, ionization energy, and electronegativity of various elements. ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.