Chemical Periodicity
... High density, in between strong and weak, brittle, fair conductors of heat. 2 Who is Dmitri Mendeleev, and what was his contribution to chemistry? He was a chemist who worked on trying to figure out the periodicity of the periodic table of the elements. He discovered the arrangement of all the ele ...
... High density, in between strong and weak, brittle, fair conductors of heat. 2 Who is Dmitri Mendeleev, and what was his contribution to chemistry? He was a chemist who worked on trying to figure out the periodicity of the periodic table of the elements. He discovered the arrangement of all the ele ...
MS Word Printable
... temperature. There are exceptions. Find three elements not in column VIII that are gas at room temperature: _______ _______ _______ Find one element that is liquid at room temperature: ________ (At# / symbol) 9. How many artificially made elements are shown on this table? (see key for symbol) ______ ...
... temperature. There are exceptions. Find three elements not in column VIII that are gas at room temperature: _______ _______ _______ Find one element that is liquid at room temperature: ________ (At# / symbol) 9. How many artificially made elements are shown on this table? (see key for symbol) ______ ...
Section 6.1 Development of the Modern Periodic Table
... called the lanthanide series and actinide series and are located at the bottom of the periodic table in the f block. ...
... called the lanthanide series and actinide series and are located at the bottom of the periodic table in the f block. ...
Ch. 6 PPT
... called the lanthanide series and actinide series and are located at the bottom of the periodic table in the f block. ...
... called the lanthanide series and actinide series and are located at the bottom of the periodic table in the f block. ...
Ch.4 Notes Powerpoint Version
... Adjusting the Periodic Table • About 40 years after Mendeleev’s table, Henry Moseley made an important change to the periodic table: ▫ Organized elements by atomic number instead of atomic mass. ▫ Elements that had not previously fit into the correct column when ordered by atomic mass were fixed. ...
... Adjusting the Periodic Table • About 40 years after Mendeleev’s table, Henry Moseley made an important change to the periodic table: ▫ Organized elements by atomic number instead of atomic mass. ▫ Elements that had not previously fit into the correct column when ordered by atomic mass were fixed. ...
Name: Chemistry A Date: Period: Unit 1 Test Review Packet
... 7. Draw the Bohr electron configuration for phosphorus (before it satisfies the Octet Rule). ...
... 7. Draw the Bohr electron configuration for phosphorus (before it satisfies the Octet Rule). ...
Periodic Properties of the Elements
... Fact: As Z (atomic number) increases ‘across a row’, the effect of shielding on the valence electrons AND their distance from the nucleus, remains ~constant. Result: Effective Nuclear Charge (Zeff) increases for each atom across every row in the Periodic table. ...
... Fact: As Z (atomic number) increases ‘across a row’, the effect of shielding on the valence electrons AND their distance from the nucleus, remains ~constant. Result: Effective Nuclear Charge (Zeff) increases for each atom across every row in the Periodic table. ...
Unit 13
... C In the early 1800s, German chemist J.W. Dobereiner observed that several elements could be classified into sets of three which he called triads. C (Li, Na, K), (Ca, Sr, Ba), (Cl, Br, I) C The elements within each triad had similar properties. C Several physical properties of the middle elements we ...
... C In the early 1800s, German chemist J.W. Dobereiner observed that several elements could be classified into sets of three which he called triads. C (Li, Na, K), (Ca, Sr, Ba), (Cl, Br, I) C The elements within each triad had similar properties. C Several physical properties of the middle elements we ...
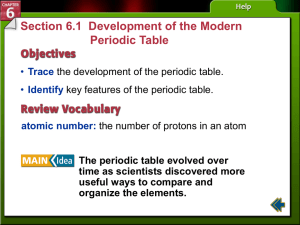
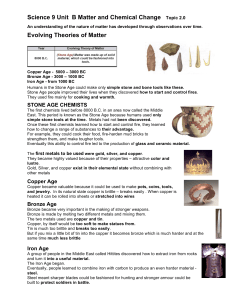
An understanding of the nature of matter has developed
... The number above the element’s symbol on the left is the atomic number. It shows how many protons are in the nucleus of one atom of the element. An oxygen atom, for example, always has eight protons. If you found six protons in an atom, the periodic table would show you that you were looking at carb ...
... The number above the element’s symbol on the left is the atomic number. It shows how many protons are in the nucleus of one atom of the element. An oxygen atom, for example, always has eight protons. If you found six protons in an atom, the periodic table would show you that you were looking at carb ...
Periodic Table ppt
... Oxygen is necessary for respiration. Many things that stink, contain sulfur (rotten eggs, garlic, skunks,etc.) ...
... Oxygen is necessary for respiration. Many things that stink, contain sulfur (rotten eggs, garlic, skunks,etc.) ...
Chapter 5: Electrons
... Section Review – page 139 Questions 1, 2, 4 and 5 End of chapter problems – page 156-157 Questions 27, 28 and 29 ...
... Section Review – page 139 Questions 1, 2, 4 and 5 End of chapter problems – page 156-157 Questions 27, 28 and 29 ...
PERIODIC TRENDS
... Standard 2: The student understands the basic principles of atomic theory. Benchmark SC.A.2.4.5: Knows that elements are arranged into groups and families based on similarities in electron structure and that their physical and chemical properties can be predicted. Task Analysis: The student… expla ...
... Standard 2: The student understands the basic principles of atomic theory. Benchmark SC.A.2.4.5: Knows that elements are arranged into groups and families based on similarities in electron structure and that their physical and chemical properties can be predicted. Task Analysis: The student… expla ...
Year9 – Chemistry Final Exam Revision Paper
... (ii) Krypton is an unreactive element in the same group of the Periodic Table as argon, but in Period 4. It has an atomic number of 36. Deduce the electronic configuration of krypton. ...
... (ii) Krypton is an unreactive element in the same group of the Periodic Table as argon, but in Period 4. It has an atomic number of 36. Deduce the electronic configuration of krypton. ...
questions on periodic classification of elements
... PERIODIC CLASSIFICATION OF ELEMENTS:Dobereiner’s Triads:- When three elements in a triad were written in the order of increasing atomic masses , the mass of the middle element was almost the average of the atomic masses of the other two elements , e.g. of triads are (1) Li Na K (2) Ca Sr Ba (3) Cl ...
... PERIODIC CLASSIFICATION OF ELEMENTS:Dobereiner’s Triads:- When three elements in a triad were written in the order of increasing atomic masses , the mass of the middle element was almost the average of the atomic masses of the other two elements , e.g. of triads are (1) Li Na K (2) Ca Sr Ba (3) Cl ...
- Schoolnet
... 19. The ionization potential of an element is the amount of energy required to remove an electron from an isolated atom or molecule. According to the periodic table, which of the following indicates the correct decreasing order of ionization potential? A. B. C. D. ...
... 19. The ionization potential of an element is the amount of energy required to remove an electron from an isolated atom or molecule. According to the periodic table, which of the following indicates the correct decreasing order of ionization potential? A. B. C. D. ...
Chapter 20 The Transition Elements
... Zinc is ued to protect or coat objects such as metal. Why? Because Zinc does not rust. Cadmium – is used also in coating metals, but is also used in rechargeable batteries. Mercury – is a silvery, liquid metal – only one at room temp. – is used in thermometers, switches, and batteries. ...
... Zinc is ued to protect or coat objects such as metal. Why? Because Zinc does not rust. Cadmium – is used also in coating metals, but is also used in rechargeable batteries. Mercury – is a silvery, liquid metal – only one at room temp. – is used in thermometers, switches, and batteries. ...
Periodictable - Trupia
... mass. Found some inconsistencies - felt that the properties were more important than the mass, so switched order. Found some gaps. Must be undiscovered elements. Predicted their properties before they were found. ...
... mass. Found some inconsistencies - felt that the properties were more important than the mass, so switched order. Found some gaps. Must be undiscovered elements. Predicted their properties before they were found. ...
vocab - SALAZAR!!
... Sentence: The Lanthanide series is the second row from the bottom. 6. Actinide series- The actinide series is much different. They are all radioactive and some are not found in nature. Sentence: The Actinide series is very reactive. 7. Noble Gas- The noble gases make a group of chemical elements wit ...
... Sentence: The Lanthanide series is the second row from the bottom. 6. Actinide series- The actinide series is much different. They are all radioactive and some are not found in nature. Sentence: The Actinide series is very reactive. 7. Noble Gas- The noble gases make a group of chemical elements wit ...
File
... Q9)Discuss the position of hydrogen in the periodic table OR –how does hydrogen resemble alkali metals and halogens in its properties Ans 9)Hydrogen has single electron in its valence shell and forms a positively charged this is similar to alkali metals such as sodium .There fore hydrogen resembles ...
... Q9)Discuss the position of hydrogen in the periodic table OR –how does hydrogen resemble alkali metals and halogens in its properties Ans 9)Hydrogen has single electron in its valence shell and forms a positively charged this is similar to alkali metals such as sodium .There fore hydrogen resembles ...
Periodic Table Intro - Chemistry Hunger Games
... Element Name and Symbol • Every element has a 1 or 2 letter symbol. • The first letter is ALWAYS capitalized. ...
... Element Name and Symbol • Every element has a 1 or 2 letter symbol. • The first letter is ALWAYS capitalized. ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.