Name Pre-Test : Atomic Structure and the Periodic Table
... Directions: Fill in the blank with the correct word from the list at the bottom of the page. Not all words from the list will be used. 1. Atomic ________________________ refers to the arrangement and number of smaller particles in an atom. 2. The ________________________ is the center or core of an ...
... Directions: Fill in the blank with the correct word from the list at the bottom of the page. Not all words from the list will be used. 1. Atomic ________________________ refers to the arrangement and number of smaller particles in an atom. 2. The ________________________ is the center or core of an ...
d) Ramsay. The idea of arranging the elements in the periodic table
... Mendeleev did not always list elements in his periodic table in order of increasing atomic mass because he grouped together elements with similar b) atomic numbers. a) properties. c) densities. ...
... Mendeleev did not always list elements in his periodic table in order of increasing atomic mass because he grouped together elements with similar b) atomic numbers. a) properties. c) densities. ...
Groups in a Periodic Table
... elements into triades. • Recognized a relationship between atomic weights and chemical properties • Triade: A set of three elements with similar properties (ex. Cl, Br, I) • Not all the known elements could be grouped into triades ...
... elements into triades. • Recognized a relationship between atomic weights and chemical properties • Triade: A set of three elements with similar properties (ex. Cl, Br, I) • Not all the known elements could be grouped into triades ...
Ionization Energy - Social Circle City Schools
... physical properties of elements and their placement on the Periodic Table (SC4b)? ...
... physical properties of elements and their placement on the Periodic Table (SC4b)? ...
periodic trend
... On one side, color and label the metals, nonmetals, and metalloids. Another name for “metalloid” is “semi-metal”. ...
... On one side, color and label the metals, nonmetals, and metalloids. Another name for “metalloid” is “semi-metal”. ...
Organizing the periodic table
... Began with Mendeleev’s Periodic Table of 63 elements. He noticed when the table was arranged in order of atomic mass, a pattern of properties became evident. The modern periodic table has over 100 elements continues to grow. ...
... Began with Mendeleev’s Periodic Table of 63 elements. He noticed when the table was arranged in order of atomic mass, a pattern of properties became evident. The modern periodic table has over 100 elements continues to grow. ...
Review sheet Atomic Structure, Electron Configuration, and Periodic
... 6. Explain why the He atom has a smaller radius than the H atom. 7. A. How many protons, electrons and neutrons in Ba2+ and Cl-1 B. Write the electron configurations for Ba2+ and Cl-1 8. Write the complete electron configuration for Uranium. Current Unit Problems: (Periodic Table) 1. In an experimen ...
... 6. Explain why the He atom has a smaller radius than the H atom. 7. A. How many protons, electrons and neutrons in Ba2+ and Cl-1 B. Write the electron configurations for Ba2+ and Cl-1 8. Write the complete electron configuration for Uranium. Current Unit Problems: (Periodic Table) 1. In an experimen ...
Periodic Table
... Group 17: the halogens are a chemical series including: fluorine , chlorine, bromine, iodine, and astatine. The word comes from Greek roots meaning "salt" and "creator". These elements are diatomic molecules in their natural form. They require one more electron to fill their outer electron shells , ...
... Group 17: the halogens are a chemical series including: fluorine , chlorine, bromine, iodine, and astatine. The word comes from Greek roots meaning "salt" and "creator". These elements are diatomic molecules in their natural form. They require one more electron to fill their outer electron shells , ...
Periodic Trends Handout
... Periodic Trends Periodic Law • Properties of elements repeat themselves periodically as they are placed in order by atomic number. Mendeleev’s Periodic Table (1869) • Arrange elements in order of increasing atomic mass. • Arrange elements in columns so that elements with similar properties are in th ...
... Periodic Trends Periodic Law • Properties of elements repeat themselves periodically as they are placed in order by atomic number. Mendeleev’s Periodic Table (1869) • Arrange elements in order of increasing atomic mass. • Arrange elements in columns so that elements with similar properties are in th ...
Unit 3 Test Review – Periodic Table (Yes, this is worth a grade!) Fill
... 1. As you go across the periodic table from left to right, ionization energy __________________ 2. As you go across the periodic table left to right, atomic size ___________________ 3. As you go across the periodic table right to left, electronegativity ___________________ 4. As you go down the peri ...
... 1. As you go across the periodic table from left to right, ionization energy __________________ 2. As you go across the periodic table left to right, atomic size ___________________ 3. As you go across the periodic table right to left, electronegativity ___________________ 4. As you go down the peri ...
Chem Ch 5 Release Test
... Evidence gathered since Mendeleev's time indicates that a better arrangement than atomic mass for elements in the periodic table is an arrangement by a. mass number. c. group number. b. atomic number. d. series number. What are the elements whose discovery added an entirely new row to Mendeleev's pe ...
... Evidence gathered since Mendeleev's time indicates that a better arrangement than atomic mass for elements in the periodic table is an arrangement by a. mass number. c. group number. b. atomic number. d. series number. What are the elements whose discovery added an entirely new row to Mendeleev's pe ...
File - Chemical Engineering
... atoms. This concept eventually became known as valency. In 1864, fellow German chemist Julius Lothar Meyer published a table of the 49 known elements arranged by valency. The table revealed that elements with similar properties often shared the same valency. English chemist John Newlands published a ...
... atoms. This concept eventually became known as valency. In 1864, fellow German chemist Julius Lothar Meyer published a table of the 49 known elements arranged by valency. The table revealed that elements with similar properties often shared the same valency. English chemist John Newlands published a ...
The Periodic Table PP
... • “When the elements are arranged according to their atomic numbers, elements with similar properties appear at regular intervals” • When elements are arranged by their atomic numbers, groups of elements begin to have ...
... • “When the elements are arranged according to their atomic numbers, elements with similar properties appear at regular intervals” • When elements are arranged by their atomic numbers, groups of elements begin to have ...
Atomic radii generally decrease along each period (row) of the
... Register for FREE to stop seeing ads ...
... Register for FREE to stop seeing ads ...
The Periodic Table
... Electronegativity • Period Trend • Increases across a period • Greater hold on electrons ...
... Electronegativity • Period Trend • Increases across a period • Greater hold on electrons ...
Periodic Trends
... 1869 – Dmitri Mendeleev published his periodic table. He had arranged it by grouping together the elements that had similar properties and by increasing atomic masses. His periodic table left empty spaces for new elements that would be discovered. ...
... 1869 – Dmitri Mendeleev published his periodic table. He had arranged it by grouping together the elements that had similar properties and by increasing atomic masses. His periodic table left empty spaces for new elements that would be discovered. ...
Organizing the periodic table
... Began with Mendeleev’s Periodic Table of 63 elements. He noticed when the table was arranged in order of atomic mass, a pattern of properties became evident. The modern periodic table has over 100 elements continues to grow. ...
... Began with Mendeleev’s Periodic Table of 63 elements. He noticed when the table was arranged in order of atomic mass, a pattern of properties became evident. The modern periodic table has over 100 elements continues to grow. ...
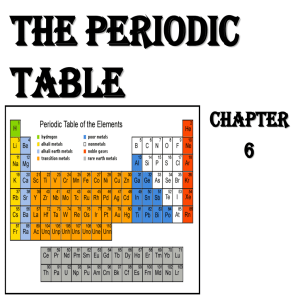
Chapter 6 Notes
... of 2 (at the time) undiscovered elements, but he predicted the properties too, and he was right! • He was very accurate in his predictions, which led the world to accept his ideas about periodicity and a logical periodic table. ...
... of 2 (at the time) undiscovered elements, but he predicted the properties too, and he was right! • He was very accurate in his predictions, which led the world to accept his ideas about periodicity and a logical periodic table. ...
File
... 6. What properties to metals, nonmetals, and metalloids have? Metals - Shiny luster, malleable, some are magnetic, good conductors of electricity and heat. Nonmetals – dull luster, brittle, nonmagnetic, insulators. Metalloids- properties of both, sometimes called semi-conductors. ...
... 6. What properties to metals, nonmetals, and metalloids have? Metals - Shiny luster, malleable, some are magnetic, good conductors of electricity and heat. Nonmetals – dull luster, brittle, nonmagnetic, insulators. Metalloids- properties of both, sometimes called semi-conductors. ...
lecture
... Mendeléev created the first accepted version of the periodic table. • He grouped elements according to their atomic number, and as he did, he found that the families had similar chemical properties. • Blank spaces were left open to add the new elements he predicted would occur. ...
... Mendeléev created the first accepted version of the periodic table. • He grouped elements according to their atomic number, and as he did, he found that the families had similar chemical properties. • Blank spaces were left open to add the new elements he predicted would occur. ...
Name________________________ Period____ Date
... 1. The horizontal rows on the periodic table are called periods_. 2. The vertical columns on the periodic table are called groups or families. 3. The Periodic Table is arranged by increasing atomic number. __. 4. Describe the location of metals, nonmetals, and metalloids on the periodic table. Metal ...
... 1. The horizontal rows on the periodic table are called periods_. 2. The vertical columns on the periodic table are called groups or families. 3. The Periodic Table is arranged by increasing atomic number. __. 4. Describe the location of metals, nonmetals, and metalloids on the periodic table. Metal ...
Chapter 5 – The Periodic Law
... • 2. He arranged the cards according to the properties and looked for trends or patterns • a. When arranged by atomic mass, properties repeated at regular intervals • b. This kind of pattern is called periodic • 1) days of the week 2) months of the year ...
... • 2. He arranged the cards according to the properties and looked for trends or patterns • a. When arranged by atomic mass, properties repeated at regular intervals • b. This kind of pattern is called periodic • 1) days of the week 2) months of the year ...
Chapter 6- The Periodic Table
... • Electronegativity is the measure of how well an atom can attract electrons from another atom to which it is bonded. • They are already bonded...what does it do to the electrons. The more electronegative it is, the more likely it is to pull off electrons of its partner. ...
... • Electronegativity is the measure of how well an atom can attract electrons from another atom to which it is bonded. • They are already bonded...what does it do to the electrons. The more electronegative it is, the more likely it is to pull off electrons of its partner. ...
Unit 2 Exam Review: Matter and its Properties This review does not
... Unit 2 Exam Review: Matter and its Properties This review does not cover everything that will be on Thursday’s exam. In order to perform at your highest level, I suggest completing the following: ...
... Unit 2 Exam Review: Matter and its Properties This review does not cover everything that will be on Thursday’s exam. In order to perform at your highest level, I suggest completing the following: ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.