Periodic Trends Name: Part 1: Summary of the Periodic Trends
... 1. Which group on the periodic table has the largest atomic radii? 2. Which group on the periodic table has the largest 1st ionization energy? 3. Which element on the periodic table has the highest and lowest electron affinity? 4. Which element on the periodic table has the largest and smallest atom ...
... 1. Which group on the periodic table has the largest atomic radii? 2. Which group on the periodic table has the largest 1st ionization energy? 3. Which element on the periodic table has the highest and lowest electron affinity? 4. Which element on the periodic table has the largest and smallest atom ...
unit iv – the periodic table
... The periodic table is the arrangement of the elements in order of their atomic numbers so that elements with similar properties fall in the same column or group. Noble Gases were added to the periodic table later - early 1900's Lanthanides - f-Block was added in the early 1900's after the identity ...
... The periodic table is the arrangement of the elements in order of their atomic numbers so that elements with similar properties fall in the same column or group. Noble Gases were added to the periodic table later - early 1900's Lanthanides - f-Block was added in the early 1900's after the identity ...
The Periodic Law Notes (Chapter 5) – Part 2
... undiscovered elements and he predicted their properties. Later elements were discovered with properties he predicted! 3. Problems with his table – a few elements did not fit – the atomic mass arrangement did not match with other similar properties. 4. Recognition – Mendeleev never received the Nobel ...
... undiscovered elements and he predicted their properties. Later elements were discovered with properties he predicted! 3. Problems with his table – a few elements did not fit – the atomic mass arrangement did not match with other similar properties. 4. Recognition – Mendeleev never received the Nobel ...
CP-Chem Ch 5 PowerPoint(The Periodic Table)
... Metals Share Many Properties • All metals are excellent conductors of electricity. • Electrical conductivity is the one property that distinguishes metals from the nonmetal elements. ...
... Metals Share Many Properties • All metals are excellent conductors of electricity. • Electrical conductivity is the one property that distinguishes metals from the nonmetal elements. ...
chemical periodicity
... Placed seven elements in each group and called it OCTAVES LAW OF OCTAVES: _______________________________________________ Did not work for all elements DMITRI MENDELEEV (1869) Listed 60+ elements in several vertical columns in order of their increasing atomic mass Noticed a regular recurrence of the ...
... Placed seven elements in each group and called it OCTAVES LAW OF OCTAVES: _______________________________________________ Did not work for all elements DMITRI MENDELEEV (1869) Listed 60+ elements in several vertical columns in order of their increasing atomic mass Noticed a regular recurrence of the ...
Know (main topic)
... divide, add, and subtract, very large and very small numbers. -describe the difference bet. the four states of matter. ...
... divide, add, and subtract, very large and very small numbers. -describe the difference bet. the four states of matter. ...
periodic trends - SpruceCreekChem
... Standard 2: The student understands the basic principles of atomic theory. Benchmark SC.A.2.4.5: Knows that elements are arranged into groups and families based on similarities in electron structure and that their physical and chemical properties can be predicted. Task Analysis: The student… expla ...
... Standard 2: The student understands the basic principles of atomic theory. Benchmark SC.A.2.4.5: Knows that elements are arranged into groups and families based on similarities in electron structure and that their physical and chemical properties can be predicted. Task Analysis: The student… expla ...
File
... lanthanides (elements 58 - 71) and actinides (elements 90 - 103). The naturally occurring rare earths are found on earth in only very small amounts. The actinides include most of the well-known elements that take part in or are produced by nuclear reactions. No element with atomic number higher than ...
... lanthanides (elements 58 - 71) and actinides (elements 90 - 103). The naturally occurring rare earths are found on earth in only very small amounts. The actinides include most of the well-known elements that take part in or are produced by nuclear reactions. No element with atomic number higher than ...
NAME - MrTestaScienceClass
... Graphing Periodic Trends Computer Activity – Academic Chemistry BACKGROUND: In 1870, Dmitri Mendeleev first proposed a new way of studying and organizing the then known 63 elements. The modern form of the table has been modified and improved many times since Mendeleev’s tables. Pioneers like Moseley ...
... Graphing Periodic Trends Computer Activity – Academic Chemistry BACKGROUND: In 1870, Dmitri Mendeleev first proposed a new way of studying and organizing the then known 63 elements. The modern form of the table has been modified and improved many times since Mendeleev’s tables. Pioneers like Moseley ...
Atomic structure and periodic table review questions What is an
... 8. An element’s atomic number represents the number of ____? 9. The atomic mass represents the sum of what two particles? 10.Elements shown on the periodic table have a ______ charge. 11.An element that has either a positive or negative charge is ___? 12.What is a positively charged element called? ...
... 8. An element’s atomic number represents the number of ____? 9. The atomic mass represents the sum of what two particles? 10.Elements shown on the periodic table have a ______ charge. 11.An element that has either a positive or negative charge is ___? 12.What is a positively charged element called? ...
PPT Periodic Families from Class
... Reading the Periodic Table • Elements are organized on the table according to their atomic number, usually found near the top of the square. • The atomic number refers to how many protons an atom of that element has. • For instance, hydrogen has 1 proton, so it’s atomic number is ...
... Reading the Periodic Table • Elements are organized on the table according to their atomic number, usually found near the top of the square. • The atomic number refers to how many protons an atom of that element has. • For instance, hydrogen has 1 proton, so it’s atomic number is ...
Bohr Model Activity
... a. Write the symbol for the element in the centre of the atom b. Below the symbol write the number of protons (For example write 15 P) and the number of neutrons (eg. 16 N). c. Write the atomic number below the drawing. d. Highlight the outer most shell with one colour. e. Use a different colour to ...
... a. Write the symbol for the element in the centre of the atom b. Below the symbol write the number of protons (For example write 15 P) and the number of neutrons (eg. 16 N). c. Write the atomic number below the drawing. d. Highlight the outer most shell with one colour. e. Use a different colour to ...
Unit 2 Periodic Table
... One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, ...
... One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, ...
Properties of Periodic Table and Periodic Trends
... Noticed elements properties repeated every 8th element when arranged by atomic mass Named this phenomenon “the Law of Octaves” Did not work for all elements ...
... Noticed elements properties repeated every 8th element when arranged by atomic mass Named this phenomenon “the Law of Octaves” Did not work for all elements ...
Power point notes - Social Circle City Schools
... series. These became known as the Actinide series. ...
... series. These became known as the Actinide series. ...
Are there atoms in the air? Why or why not?
... Elements, Compounds and Mixtures! Let’s Review: Element: pure substance that cannot be separated into simpler substance by physical or chemical means. An atom is the smallest part of an element. Atoms are made of protons, neutrons, and electrons. All things on Earth, except energy, are made of atom ...
... Elements, Compounds and Mixtures! Let’s Review: Element: pure substance that cannot be separated into simpler substance by physical or chemical means. An atom is the smallest part of an element. Atoms are made of protons, neutrons, and electrons. All things on Earth, except energy, are made of atom ...
The Periodic Table
... Elements were listed in columns so that those with similar properties were side by side. ...
... Elements were listed in columns so that those with similar properties were side by side. ...
family includes a non-metal
... One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, ...
... One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, ...
Review of atomic structure and the periodic table
... For me too, the periodic table was a passion…. As a boy, I stood in front of the [Science Museum, London] display for hours, thinking how wonderful it was that each of these metal foils and jars of gas had its own distinct personality. Freeman Dyson ...
... For me too, the periodic table was a passion…. As a boy, I stood in front of the [Science Museum, London] display for hours, thinking how wonderful it was that each of these metal foils and jars of gas had its own distinct personality. Freeman Dyson ...
Chapter 6 - HCC Learning Web
... • In 1829, the German chemist J.W. Döbereiner observed that several elements could be classified into groups of three, or triads. • All three elements in a triad showed very similar chemical properties and an orderly trend in physical properties. ...
... • In 1829, the German chemist J.W. Döbereiner observed that several elements could be classified into groups of three, or triads. • All three elements in a triad showed very similar chemical properties and an orderly trend in physical properties. ...
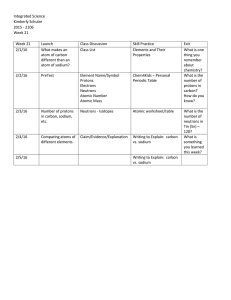
Week 21 Lessons - Highline Public Schools
... - Research the squares on the periodic table and write into your own personal periodic table. (Periodic Table Basics) - Use Chem4Kids.com to find all the relevent information. Go to Element List, choose element, “More about orbitals and compounds.” Bohr Diagram is located at the end of More abou ...
... - Research the squares on the periodic table and write into your own personal periodic table. (Periodic Table Basics) - Use Chem4Kids.com to find all the relevent information. Go to Element List, choose element, “More about orbitals and compounds.” Bohr Diagram is located at the end of More abou ...
An element
... grouped with oxygen and sulphur. • Newlands tried to force all of the known elements into the table instead of leaving gaps for elements not yet discovered example: Li and Ag in same group. ...
... grouped with oxygen and sulphur. • Newlands tried to force all of the known elements into the table instead of leaving gaps for elements not yet discovered example: Li and Ag in same group. ...
Chapter 8 Reading Guide Name: AP Chemistry 2016
... 24. Sketch a periodic table. Identify the location of atoms that are filling their last electrons in s, p, d, and f orbitals. ...
... 24. Sketch a periodic table. Identify the location of atoms that are filling their last electrons in s, p, d, and f orbitals. ...
alkaline earth metals
... • Often conduct electricity and heat well • Can be easily shaped and drawn into wire ...
... • Often conduct electricity and heat well • Can be easily shaped and drawn into wire ...
How to Read the Periodic Table
... The Periodic table is designed to help you predict what an element's physical and chemical properties are. You can also predict what elements will bond with each other. First, let's look at the columns and rows of the periodic table. ...
... The Periodic table is designed to help you predict what an element's physical and chemical properties are. You can also predict what elements will bond with each other. First, let's look at the columns and rows of the periodic table. ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.