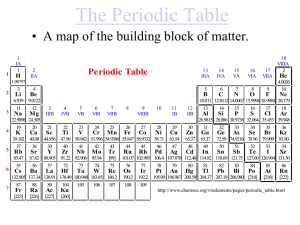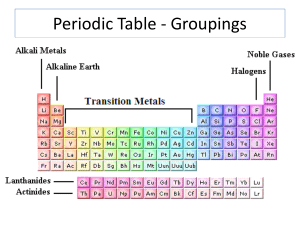
Periodic Table
... • All atoms what to have a balanced charge but they also want to have a full valance shell. Atoms will often take, loose or share electrons on order to fill the valance. All atoms that have the same # of electrons behave in a similar fashion. The atoms with fewer electrons on the valance and/or the ...
... • All atoms what to have a balanced charge but they also want to have a full valance shell. Atoms will often take, loose or share electrons on order to fill the valance. All atoms that have the same # of electrons behave in a similar fashion. The atoms with fewer electrons on the valance and/or the ...
Chapter 4 Notes - Riverton High School
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
Chapter 7. Periodic Properties of the Elements.
... 7.1 Development of The Periodic Table. The number of known elements in 1800 was 31, but had increased to 63 by 1865. In 1869 Mendeleev and Lothar Meyer published almost identical classification schemes. Both noticed that similar chemical properties recurred when elements were placed in order of inc ...
... 7.1 Development of The Periodic Table. The number of known elements in 1800 was 31, but had increased to 63 by 1865. In 1869 Mendeleev and Lothar Meyer published almost identical classification schemes. Both noticed that similar chemical properties recurred when elements were placed in order of inc ...
Document
... Instructions: You have 2 minutes to complete this task. This is individual work. You are seated and silent. The Periodic Table is arranged according to the Periodic Law. The Periodic Law states that when elements are arranged in order of increasing atomic number, their physical and chemical properti ...
... Instructions: You have 2 minutes to complete this task. This is individual work. You are seated and silent. The Periodic Table is arranged according to the Periodic Law. The Periodic Law states that when elements are arranged in order of increasing atomic number, their physical and chemical properti ...
sample
... He published his results in 1668. In one experiment, Mayow put a lighted candle in a dish of water and covered it with an upturned jar. The flame eventually went out, and water rose a little inside the jar. If he put a mouse inside instead of a candle, it eventually suffocated and water rose again. ...
... He published his results in 1668. In one experiment, Mayow put a lighted candle in a dish of water and covered it with an upturned jar. The flame eventually went out, and water rose a little inside the jar. If he put a mouse inside instead of a candle, it eventually suffocated and water rose again. ...
California Standards Practice
... 25. The periodic table is organized into blocks representing the energy sublevel being filled with valence electrons. In the periodic table, which sequence lists the blocks in s-p-d-f order? A. W, Y, X, Z B. X, Y, Z, W C. Y, W, Z, X D. Y, Z, W, X 26. In the periodic table, which block represents the ...
... 25. The periodic table is organized into blocks representing the energy sublevel being filled with valence electrons. In the periodic table, which sequence lists the blocks in s-p-d-f order? A. W, Y, X, Z B. X, Y, Z, W C. Y, W, Z, X D. Y, Z, W, X 26. In the periodic table, which block represents the ...
Click Here
... Johann Dobereiner noticed that the middle element of each of the Triads had an atomic weight about half way between the atomic weights of the other two. Also the properties of the middle element were in between those of the other two members. Since Dobereiner’s relationship, referred to as the Law o ...
... Johann Dobereiner noticed that the middle element of each of the Triads had an atomic weight about half way between the atomic weights of the other two. Also the properties of the middle element were in between those of the other two members. Since Dobereiner’s relationship, referred to as the Law o ...
AtomTest
... Select the pen tool from the box on the bottom left hand corner of this page and use it to draw a line from each label to its place on the periodic table. When you are done select automatic pointer and click the bottom right arrow to check ...
... Select the pen tool from the box on the bottom left hand corner of this page and use it to draw a line from each label to its place on the periodic table. When you are done select automatic pointer and click the bottom right arrow to check ...
The perfect K-12 presentation ever (replace this with your title)
... Main Sequence Stars, such as Red Giants, derive their energy from hydrogen fusion. ...
... Main Sequence Stars, such as Red Giants, derive their energy from hydrogen fusion. ...
ch3 - ChemistryVCE
... The compound commonly formed between oxygen and hydrogen has the formula H2O. As sulfur is in the same group as oxygen, we would predict the compound between sulfur and hydrogen to have the formula H2S. Q15. Consider elements that make up the following compounds. Which of the compounds are likely to ...
... The compound commonly formed between oxygen and hydrogen has the formula H2O. As sulfur is in the same group as oxygen, we would predict the compound between sulfur and hydrogen to have the formula H2S. Q15. Consider elements that make up the following compounds. Which of the compounds are likely to ...
ch3 - sscyr11chemistry
... The compound commonly formed between oxygen and hydrogen has the formula H2O. As sulfur is in the same group as oxygen, we would predict the compound between sulfur and hydrogen to have the formula H2S. Q15. Consider elements that make up the following compounds. Which of the compounds are likely to ...
... The compound commonly formed between oxygen and hydrogen has the formula H2O. As sulfur is in the same group as oxygen, we would predict the compound between sulfur and hydrogen to have the formula H2S. Q15. Consider elements that make up the following compounds. Which of the compounds are likely to ...
Periodic Properties
... – Ionization energy – the energy that is required to remove an e- from an atom – Ion – atom which has gained or lost electrons • Cation – (+) charged ion (lost e-) • Anion – (-) charged ion (gained e-) ...
... – Ionization energy – the energy that is required to remove an e- from an atom – Ion – atom which has gained or lost electrons • Cation – (+) charged ion (lost e-) • Anion – (-) charged ion (gained e-) ...
Study Guide for Electrons Mini-Test - seys
... at the time. He collected information on the properties of those 63 elements and grouped them using data that many other scientists had collected about the properties of each of the elements. When he arranged the elements according to their atomic masses and their physical and chemical properties, h ...
... at the time. He collected information on the properties of those 63 elements and grouped them using data that many other scientists had collected about the properties of each of the elements. When he arranged the elements according to their atomic masses and their physical and chemical properties, h ...
Periodic Law
... – Horizontal rows of elements with atomic mass and similar electron configurations. ©Bires, 2002 ...
... – Horizontal rows of elements with atomic mass and similar electron configurations. ©Bires, 2002 ...
Chemistry Definitions
... Mass is the measure of the amount of matter. Matter is anything that has mass and takes up space. An Atom is the smallest unit of an element that has the properties of that element. An Element is a pure substance made of only one type kind of atom. A Compound is a substance that is made from the ato ...
... Mass is the measure of the amount of matter. Matter is anything that has mass and takes up space. An Atom is the smallest unit of an element that has the properties of that element. An Element is a pure substance made of only one type kind of atom. A Compound is a substance that is made from the ato ...
The Periodic Table
... have properties of both metals and non-metals. can be shiny or dull. conduct heat and electricity better than non-metals but not as well as metals. ductile and malleable. ...
... have properties of both metals and non-metals. can be shiny or dull. conduct heat and electricity better than non-metals but not as well as metals. ductile and malleable. ...
periodic table
... Russian chemist, searched for a way to organize the elements. • When he arranged all the elements known at that time in order of increasing atomic masses, he discovered a pattern. ...
... Russian chemist, searched for a way to organize the elements. • When he arranged all the elements known at that time in order of increasing atomic masses, he discovered a pattern. ...
The Upper Limit of the Periodic Table of Elements Points out to the
... multaneously the sub-Group “b” contains copper, silver and Chemical Similarity”, in terms of the dependence of each gold which have not these properties but possess excellent atomic mass on the number of the corresponding element, electric conductivity. Besides gold, silver and platinum, met- has be ...
... multaneously the sub-Group “b” contains copper, silver and Chemical Similarity”, in terms of the dependence of each gold which have not these properties but possess excellent atomic mass on the number of the corresponding element, electric conductivity. Besides gold, silver and platinum, met- has be ...
Atomic Structure Webquest Links
... 1. What were Leucippus and Democritus ideas regarding matter? 2. Describe what these philosophers thought the atom looked like? 3. How were the ideas of these two men received by Aristotle, and what was the result on the progress of atomic theory for the next couple thousand years? Alchemists: Go to ...
... 1. What were Leucippus and Democritus ideas regarding matter? 2. Describe what these philosophers thought the atom looked like? 3. How were the ideas of these two men received by Aristotle, and what was the result on the progress of atomic theory for the next couple thousand years? Alchemists: Go to ...
Chapter 6 lecture 2013
... The Periodic Law • In the modern periodic table, elements are arranged in order of increasing atomic number. • Organized into columns and rows. • 7 horizontal rows correspond to the energy levels found outside an atom’s nucleus. ...
... The Periodic Law • In the modern periodic table, elements are arranged in order of increasing atomic number. • Organized into columns and rows. • 7 horizontal rows correspond to the energy levels found outside an atom’s nucleus. ...
Chemistry Unit 2 - Finding Patterns
... More often, however, the changes that take place in the body of scientific knowledge are small modifications of prior knowledge. Major shifts in scientific views typically occur after the observation of a new phenomenon or an insightful interpretation of existing data by an individual or research gr ...
... More often, however, the changes that take place in the body of scientific knowledge are small modifications of prior knowledge. Major shifts in scientific views typically occur after the observation of a new phenomenon or an insightful interpretation of existing data by an individual or research gr ...
D. - Taylor County Schools
... Development of the Periodic Table (cont.) • Meyer and Mendeleev both demonstrated a connection between atomic mass and elemental properties. • When creating his periodic table, Mendeleev left blank spaces for elements that had not yet been discovered. • Using periodic properties of the other elemen ...
... Development of the Periodic Table (cont.) • Meyer and Mendeleev both demonstrated a connection between atomic mass and elemental properties. • When creating his periodic table, Mendeleev left blank spaces for elements that had not yet been discovered. • Using periodic properties of the other elemen ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.