Periodic Trends Worksheet
... 18. Based on the concept of periodic trends, answer the following questions for these atoms: Li, Be, Mg, Na. Be able to defend your answers. a. Which element has the lowest electronegativity? _________________________________ b. Which element has the least metallic character? ______________________ ...
... 18. Based on the concept of periodic trends, answer the following questions for these atoms: Li, Be, Mg, Na. Be able to defend your answers. a. Which element has the lowest electronegativity? _________________________________ b. Which element has the least metallic character? ______________________ ...
periodic trends worksheet
... 18. Based on the concept of periodic trends, answer the following questions for these atoms: Li, Be, Mg, Na. Be able to defend your answers. a. Which element has the lowest electronegativity? _________________________________ b. Which element has the least metallic character? ______________________ ...
... 18. Based on the concept of periodic trends, answer the following questions for these atoms: Li, Be, Mg, Na. Be able to defend your answers. a. Which element has the lowest electronegativity? _________________________________ b. Which element has the least metallic character? ______________________ ...
Diff Chemistry
... The above graph is a plot of the relative atomic radius and relative ionization energy as a function of the atomic number. Relative means that adjustments were made so that both plots fit on the same graph and are both clearly visible. ...
... The above graph is a plot of the relative atomic radius and relative ionization energy as a function of the atomic number. Relative means that adjustments were made so that both plots fit on the same graph and are both clearly visible. ...
Periodic Table Trends
... The larger the atom, the more readily it ionises and the more reactive it is. The reactivity of the metals in Group II therefore, increases as one moves down the group as does their metallic nature. Beryllium (Be) is the least reactive element and radium (Ra) is the most reactive element. ...
... The larger the atom, the more readily it ionises and the more reactive it is. The reactivity of the metals in Group II therefore, increases as one moves down the group as does their metallic nature. Beryllium (Be) is the least reactive element and radium (Ra) is the most reactive element. ...
The Periodic Table and Chemical Properties
... a Canadian researcher who worked with Ernest Rutherford. She was one of the early scientists who found that a gas being released from the element radium was in fact a new element: radon. ...
... a Canadian researcher who worked with Ernest Rutherford. She was one of the early scientists who found that a gas being released from the element radium was in fact a new element: radon. ...
Atoms and Elements: Are they Related?
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...
Graphing Trends in the Periodic Table
... 2. For elements in Family IA, make a graph of atomic radius as a function of atomic number. On the same graph, use a different color to do the same for elements in Family HA. Label the graph. 3. For elements 3—20, make a graph of the energy required to remove the easiest electron as a function of at ...
... 2. For elements in Family IA, make a graph of atomic radius as a function of atomic number. On the same graph, use a different color to do the same for elements in Family HA. Label the graph. 3. For elements 3—20, make a graph of the energy required to remove the easiest electron as a function of at ...
3. classification of elements and periodicity in properties
... Johann Dobereiner classified elements into small groups each containing three elements. These small groups were called triads. ...
... Johann Dobereiner classified elements into small groups each containing three elements. These small groups were called triads. ...
The Periodic Table and The Periodic Law
... Table 3. Dimitri Mendeleev – is the person credited with making the 1st periodic table leaving spaces for the predicted elements in future. (It was based on the atomic masses) 4. Henry Mosley – he then rearranged the periodic table based on atomic numbers and their characteristics, it is used till t ...
... Table 3. Dimitri Mendeleev – is the person credited with making the 1st periodic table leaving spaces for the predicted elements in future. (It was based on the atomic masses) 4. Henry Mosley – he then rearranged the periodic table based on atomic numbers and their characteristics, it is used till t ...
Elements, Periodic Trends and Lewis Dot Diagrams
... • Know how to figure out the number of valence electrons for an atom • Understand electronegaJvity; use this to predict formaJon of compounds in general terms • State the octet rule – name and define ...
... • Know how to figure out the number of valence electrons for an atom • Understand electronegaJvity; use this to predict formaJon of compounds in general terms • State the octet rule – name and define ...
Periodic Trends
... Going down the periodic table, atomic radius tends get bigger within a group. Even though the number of protons is increasing, new energy levels are added as you move down which move the valence electrons further away. Also, the additional orbitals between the nucleus and the outer electrons are occ ...
... Going down the periodic table, atomic radius tends get bigger within a group. Even though the number of protons is increasing, new energy levels are added as you move down which move the valence electrons further away. Also, the additional orbitals between the nucleus and the outer electrons are occ ...
Homework Answers - Chemistry from AZ
... vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have similar chemical properties. Note there are some variations in the transition metals. Group 1 Alkali Metals: hydrogen is NOT a member; good conduc ...
... vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have similar chemical properties. Note there are some variations in the transition metals. Group 1 Alkali Metals: hydrogen is NOT a member; good conduc ...
Elements and the Periodic Table
... Organizing the Elements Metals Nonmetals and Metalloids Elements From Stardust ...
... Organizing the Elements Metals Nonmetals and Metalloids Elements From Stardust ...
Periodic Table Student Outline
... Now he had a new Periodic Law (“Elements arranged according to the value of their atomic weights present a clear periodicity of properties”) that described one pattern for all 63 elements. Where Mendeleeev’s table had blank spaces, he correctly predicted the weights and chemical behaviors of some mi ...
... Now he had a new Periodic Law (“Elements arranged according to the value of their atomic weights present a clear periodicity of properties”) that described one pattern for all 63 elements. Where Mendeleeev’s table had blank spaces, he correctly predicted the weights and chemical behaviors of some mi ...
Atoms and Elements: Are they Related?
... • Which parts of an atom make up the mass of the atom? • Elements are made up of? • The element lead is made up of what kind of atoms? ...
... • Which parts of an atom make up the mass of the atom? • Elements are made up of? • The element lead is made up of what kind of atoms? ...
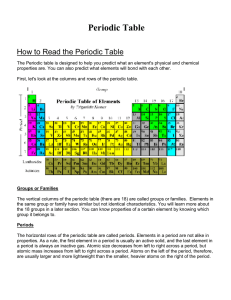
How to Read the Periodic Table
... How to Read the Periodic Table: 1. What are the vertical columns of the Periodic Table called? 2. What are the horizontal rows of the Periodic Table called? 3. How many groups/families are there on the Periodic Table? 4. How many periods are there on the Periodic Table? 5. Describe a trend in the Pe ...
... How to Read the Periodic Table: 1. What are the vertical columns of the Periodic Table called? 2. What are the horizontal rows of the Periodic Table called? 3. How many groups/families are there on the Periodic Table? 4. How many periods are there on the Periodic Table? 5. Describe a trend in the Pe ...
Daily 40 no. – 15 John Newlands
... John Newlands was born in London in 1837 and died in 1898. He noticed that, when the elements are arranged in consecutive order of their atomic numbers, patterns emerge and thus proposed the Law of Octaves. He predicted the existence of germanium and studied at the Royal College of Chemistry. -Alann ...
... John Newlands was born in London in 1837 and died in 1898. He noticed that, when the elements are arranged in consecutive order of their atomic numbers, patterns emerge and thus proposed the Law of Octaves. He predicted the existence of germanium and studied at the Royal College of Chemistry. -Alann ...
Electron Configurations and Periodic Properties Name
... Electron Configurations and Periodic Properties Name:___________________ Guided Inquiry Period:____ Objective: You have learned how the atoms are arranged and how to determine the electron configurations for atoms. Now we need to look at the relationship between electron configurations and periodic ...
... Electron Configurations and Periodic Properties Name:___________________ Guided Inquiry Period:____ Objective: You have learned how the atoms are arranged and how to determine the electron configurations for atoms. Now we need to look at the relationship between electron configurations and periodic ...
chemistry, grade 11, university preparation, sch3u
... If a liquid does catch fire accidentally in a small container, try covering the mouth of the container with a book or a damp cloth. It should go out because it has no oxygen to keep it going. If it does not, stand well back and call your teacher. Never try to blow it out—it doesn’t work, and usually ...
... If a liquid does catch fire accidentally in a small container, try covering the mouth of the container with a book or a damp cloth. It should go out because it has no oxygen to keep it going. If it does not, stand well back and call your teacher. Never try to blow it out—it doesn’t work, and usually ...
Atomic Structure and the Periodic Table of Elements: The Secret
... number of protons, which equals the number of electrons, so the positive and negative charges cancel, and give the element’s atom an overall neutral charge. An abbreviated form of referring to the element is through its Atomic Symbol (Zn), though the actual name of the element is always given (Zinc) ...
... number of protons, which equals the number of electrons, so the positive and negative charges cancel, and give the element’s atom an overall neutral charge. An abbreviated form of referring to the element is through its Atomic Symbol (Zn), though the actual name of the element is always given (Zinc) ...
Unit 4 Pack
... 13. As you go from left to right across the periodic table the elements go from (metals/nonmetals) to (M /NM) 14. Group 17 is called __________ 15. The most active element in group 17 is ____________ 16. Group 18 elements are called ____________ 17. What sublevels are filling across the transition e ...
... 13. As you go from left to right across the periodic table the elements go from (metals/nonmetals) to (M /NM) 14. Group 17 is called __________ 15. The most active element in group 17 is ____________ 16. Group 18 elements are called ____________ 17. What sublevels are filling across the transition e ...
The Periodic Table
... • Presented the first valid periodic table of elements to the Academy of Sciences in 1869 • Grouped elements in ascending order by atomic mass and by similarities in properties • left gaps for elements not yet discovered ...
... • Presented the first valid periodic table of elements to the Academy of Sciences in 1869 • Grouped elements in ascending order by atomic mass and by similarities in properties • left gaps for elements not yet discovered ...
Periodicity of Elements
... This puute uses the chemical symbols and their corresponding names. Only those elements which are metals are included. Since this is a rich puzzle indeed, two very valuable metals are used in each column. Fill in the proper word on the puzzle and on the lines provided by the chemical symbol. .ACROSS ...
... This puute uses the chemical symbols and their corresponding names. Only those elements which are metals are included. Since this is a rich puzzle indeed, two very valuable metals are used in each column. Fill in the proper word on the puzzle and on the lines provided by the chemical symbol. .ACROSS ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.