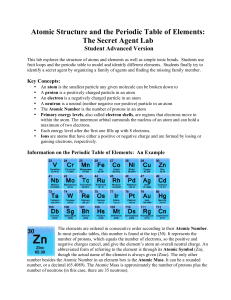
Atomic Structure and the Periodic Table of Elements: The Secret
... Today, the elements of the Periodic Table are arranged by atomic number, which also indicates the number of protons in an atom (see Periodic Table Tutorial above). Neutrons are uncharged particles in the atom’s nucleus that only affect the overall weight of the atom, not the charge. The Periodic Tab ...
... Today, the elements of the Periodic Table are arranged by atomic number, which also indicates the number of protons in an atom (see Periodic Table Tutorial above). Neutrons are uncharged particles in the atom’s nucleus that only affect the overall weight of the atom, not the charge. The Periodic Tab ...
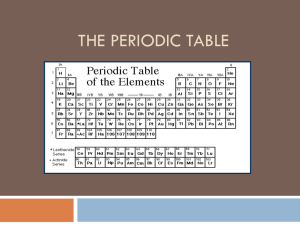
The Periodic Table of Elements
... *There is in the atom a fundamental quantity which increases by regular steps as we pass from each element to the next. This quantity can only be the charge on the central positive nucleus. His research was halted when the British government sent him to serve as a foot soldier in WWI. He was killed ...
... *There is in the atom a fundamental quantity which increases by regular steps as we pass from each element to the next. This quantity can only be the charge on the central positive nucleus. His research was halted when the British government sent him to serve as a foot soldier in WWI. He was killed ...
8.3 Metals - UNSW Chemistry
... chemical reactions could be discussed. For example: all three elements form –1 anions. The binary hydrogen compounds of all three (i.e. HCl, HBr, HI) are acidic. Döbereiner’s system was inadequate because a great many elements did not seem to belong to any triad, and the chemistry of many elements w ...
... chemical reactions could be discussed. For example: all three elements form –1 anions. The binary hydrogen compounds of all three (i.e. HCl, HBr, HI) are acidic. Döbereiner’s system was inadequate because a great many elements did not seem to belong to any triad, and the chemistry of many elements w ...
Periodicity - Teach-n-Learn-Chem
... FAMILIES OF ELEMENTS Dimitri Mendeleev invented the periodic table ...
... FAMILIES OF ELEMENTS Dimitri Mendeleev invented the periodic table ...
File
... Mendeleev published the first periodic table. In the periodic table, the properties of the elements repeat in each period, or row, of the table. Mendeleev left three blank spaces in the table. He predicted that these spaces would be filled by elements that had not yet been discovered. He even predic ...
... Mendeleev published the first periodic table. In the periodic table, the properties of the elements repeat in each period, or row, of the table. Mendeleev left three blank spaces in the table. He predicted that these spaces would be filled by elements that had not yet been discovered. He even predic ...
PERIODIC TABLE
... 2. During the 1800s many more were discovered as scientists used electricity to separate compounds and newly developed instruments, such as the spectrometer, to identify the elements. By 1870, there were approximately 70 known elements. 3. Chemists were overwhelmed by the volume of new information a ...
... 2. During the 1800s many more were discovered as scientists used electricity to separate compounds and newly developed instruments, such as the spectrometer, to identify the elements. By 1870, there were approximately 70 known elements. 3. Chemists were overwhelmed by the volume of new information a ...
Atoms and The Periodic Table
... naturalists that ever lived, explained that all matter was composed of four earth elements: earth, air, fire, and water. Combinations of these four elements made up everything in the universe. Rearrangement of these elements would result, according to Aristotle, in the formation of different or new ...
... naturalists that ever lived, explained that all matter was composed of four earth elements: earth, air, fire, and water. Combinations of these four elements made up everything in the universe. Rearrangement of these elements would result, according to Aristotle, in the formation of different or new ...
20161025131513
... o # of known elements at his timeo what inspired his approach to the periodic tableo made a “deck of cards” o what patterns did he noticeo what was his final arrangement of the periodic tableo what was missing in his tableo how did he predict undiscovered elementso was he the first to make a periodi ...
... o # of known elements at his timeo what inspired his approach to the periodic tableo made a “deck of cards” o what patterns did he noticeo what was his final arrangement of the periodic tableo what was missing in his tableo how did he predict undiscovered elementso was he the first to make a periodi ...
Periodic Table - Chemistry R: 4(AE)
... • By 1860 more than 60 elements had been discovered. • Chemists learned about the new elements by reacting them with other elements to form new compounds. • In 1865, John Newlands, an English chemist, arranged the known elements according to their properties in order of increasing atomic mass. • The ...
... • By 1860 more than 60 elements had been discovered. • Chemists learned about the new elements by reacting them with other elements to form new compounds. • In 1865, John Newlands, an English chemist, arranged the known elements according to their properties in order of increasing atomic mass. • The ...
Periodic Law
... • Periodic Law – chemical and physical properties of elements are periodic functions of their atomic numbers. – The elements in the periodic table are arranged according to Periodic Law – Periodic Law shows certain trends in the properties of elements … Slide 3 ...
... • Periodic Law – chemical and physical properties of elements are periodic functions of their atomic numbers. – The elements in the periodic table are arranged according to Periodic Law – Periodic Law shows certain trends in the properties of elements … Slide 3 ...
Chemical evidence of the transmutation of elements
... undertake this work properly, fairly large quantities will be required. This will be achieved by the use of artificially accelereated projectiles. Equipment suitable for this is already in existence in several countries. In France, we have built two installations with which we have recently been abl ...
... undertake this work properly, fairly large quantities will be required. This will be achieved by the use of artificially accelereated projectiles. Equipment suitable for this is already in existence in several countries. In France, we have built two installations with which we have recently been abl ...
The 7 Secrets of the Periodic Table
... The periodic table also accounts for the electron configuration of an electron. The first two families represent the "s" orbitals and the next six families represent the "p" orbitals. It is imperative that you take the time to understand how to write an electron configuration ...
... The periodic table also accounts for the electron configuration of an electron. The first two families represent the "s" orbitals and the next six families represent the "p" orbitals. It is imperative that you take the time to understand how to write an electron configuration ...
Introduction to Mendeleev*s Periodic Table of Elements
... Mendeleev’s Periodic Table of Elements •Only 63 elements known at time •He left spaces on the table for undiscovered elements. ...
... Mendeleev’s Periodic Table of Elements •Only 63 elements known at time •He left spaces on the table for undiscovered elements. ...
Chapt 6: Arrangement of the Elements
... observed that several elements could be classified into groups of three, or triads. • All three elements in a triad showed very similar chemical properties and an orderly trend in physical properties. © 2011 Pearson Education, Inc. ...
... observed that several elements could be classified into groups of three, or triads. • All three elements in a triad showed very similar chemical properties and an orderly trend in physical properties. © 2011 Pearson Education, Inc. ...
chemistry chapter 11 & 12
... •Horizontal rows have similar chemical properties •Missing Elements -Gaps existed in Mendeleev’s table •Mendeleev predicted properties of the “yet to be discovered” elements ...
... •Horizontal rows have similar chemical properties •Missing Elements -Gaps existed in Mendeleev’s table •Mendeleev predicted properties of the “yet to be discovered” elements ...
Chapter 13
... repeated when they were arranged according to increasing atomic mass in groups of eight He called this the arrangement the law of octaves Similar to musical scale that repeats every eighth note Law only works up to Ca ...
... repeated when they were arranged according to increasing atomic mass in groups of eight He called this the arrangement the law of octaves Similar to musical scale that repeats every eighth note Law only works up to Ca ...
Standards Practice
... 29. By measuring the effects of magnetic and electrical fields on cathode rays, J.J. Thomson discovered that these particles A. had no mass. B. were heavier than hydrogen atoms. C. were smaller than hydrogen atoms. D. were easily broken apart. 30. Robert Millikan dropped negatively charged oil dropl ...
... 29. By measuring the effects of magnetic and electrical fields on cathode rays, J.J. Thomson discovered that these particles A. had no mass. B. were heavier than hydrogen atoms. C. were smaller than hydrogen atoms. D. were easily broken apart. 30. Robert Millikan dropped negatively charged oil dropl ...
Solutions Tutorial 8
... First, we note that this is an isoelectronic series of ions, with all ions having 36 electrons. In such a series, size decreases as the nuclear charge (atomic number) of the ion increases. The atomic numbers of the ions are As (33), Se (34), Br (35), Rb (37), and Sr (38). Reasoning : Consider the tr ...
... First, we note that this is an isoelectronic series of ions, with all ions having 36 electrons. In such a series, size decreases as the nuclear charge (atomic number) of the ion increases. The atomic numbers of the ions are As (33), Se (34), Br (35), Rb (37), and Sr (38). Reasoning : Consider the tr ...
The Periodic Table
... 19, Elements within a group have a similar number of 20. Elements across a series have the same number of. 21. A colored ion generally indicates a 22. As you go down a group, the elements generally become ( more / less) metallic. 23. The majority of elements in the periodic table are (metals / nonme ...
... 19, Elements within a group have a similar number of 20. Elements across a series have the same number of. 21. A colored ion generally indicates a 22. As you go down a group, the elements generally become ( more / less) metallic. 23. The majority of elements in the periodic table are (metals / nonme ...
General Principles of Chemistry – CHEM110 Tutorial 8 – 9 and 11
... First, we note that this is an isoelectronic series of ions, with all ions having 36 electrons. In such a series, size decreases as the nuclear charge (atomic number) of the ion increases. The atomic numbers of the ions are As (33), Se (34), Br (35), Rb (37), and Sr (38). Reasoning : Consider the tr ...
... First, we note that this is an isoelectronic series of ions, with all ions having 36 electrons. In such a series, size decreases as the nuclear charge (atomic number) of the ion increases. The atomic numbers of the ions are As (33), Se (34), Br (35), Rb (37), and Sr (38). Reasoning : Consider the tr ...
C1 - Powerpoint - tonyconnett.com
... 2. Group 1 atoms want to loose an electron, do you think it would be easier to remove an electron from Lithium or Cs? 3. What is the most reactive element in group 1? 4. Group 7 atoms want to gain an electron, which atom would most strongly attract an electron to the ‘gap’ in the outer shell? 5. Whi ...
... 2. Group 1 atoms want to loose an electron, do you think it would be easier to remove an electron from Lithium or Cs? 3. What is the most reactive element in group 1? 4. Group 7 atoms want to gain an electron, which atom would most strongly attract an electron to the ‘gap’ in the outer shell? 5. Whi ...
Entry Task
... • Nonmetals– elements on the right of the periodic table – Properties are opposite of metals – Their properties vary more from element to element than the properties of metals – All are gas in their natural state at room temperature except for bromine which is a liquid – Poor conductors of heat and ...
... • Nonmetals– elements on the right of the periodic table – Properties are opposite of metals – Their properties vary more from element to element than the properties of metals – All are gas in their natural state at room temperature except for bromine which is a liquid – Poor conductors of heat and ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.