CST Review Part 2
... of gases. c. Students know how to apply the gas laws to relations between the pressure, temperature, and volume of any amount of an ideal gas or any mixture of ideal gases. d. Students know the values and meanings of standard temperature and pressure (STP). e. Students know how to convert between th ...
... of gases. c. Students know how to apply the gas laws to relations between the pressure, temperature, and volume of any amount of an ideal gas or any mixture of ideal gases. d. Students know the values and meanings of standard temperature and pressure (STP). e. Students know how to convert between th ...
Name - cloudfront.net
... 44. Standard conditions of temperature and pressure for a thermochemical equation are __ and __kPa. 45. If heat is released by a chemical system, an equal amount of heat will be ____. 46. By what quantity must the heat capacity (J/oC) of an object be divided to obtain the specific heat (J/goC) of th ...
... 44. Standard conditions of temperature and pressure for a thermochemical equation are __ and __kPa. 45. If heat is released by a chemical system, an equal amount of heat will be ____. 46. By what quantity must the heat capacity (J/oC) of an object be divided to obtain the specific heat (J/goC) of th ...
Physics, Chapter 17: The Phases of Matter
... The vaporization curve is not indefinite in extent; it has both an upper and a lower limit. The upper limit is knowp as the critical point, shown as point A in Figure 17-2. The temperature and pressure at the critical point are known as the critical temperature and critical pressure. If a glass vial ...
... The vaporization curve is not indefinite in extent; it has both an upper and a lower limit. The upper limit is knowp as the critical point, shown as point A in Figure 17-2. The temperature and pressure at the critical point are known as the critical temperature and critical pressure. If a glass vial ...
Document
... Enthalpies of transition • For a chemical reaction, if we define ”standard” conditions for reagents g and p products the change g in the thermodynamic (state) function will not depend on the path or intermediate steps. p p • Hess’s law: Standard enthalpy (entropy, Gibbs free energy etc.) of an over ...
... Enthalpies of transition • For a chemical reaction, if we define ”standard” conditions for reagents g and p products the change g in the thermodynamic (state) function will not depend on the path or intermediate steps. p p • Hess’s law: Standard enthalpy (entropy, Gibbs free energy etc.) of an over ...
Chem A Week 2 Matter Notes
... V. Two Categories of Matter A. Pure Substances - a substance whose component parts are always uniform matter. They have the same phase and properties throughout. They can not be physically ...
... V. Two Categories of Matter A. Pure Substances - a substance whose component parts are always uniform matter. They have the same phase and properties throughout. They can not be physically ...
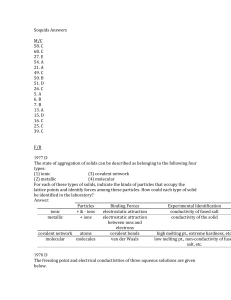
Soquids Answers M/C 58. C 68. C 27. E 54. A 21. A 49. C 50. B 51
... Using principles of chemical bonding and/or intermolecular forces, explain each of the following. (a) Xenon has a higher boiling point than neon has. (b) Solid copper is an excellent conductor of electricity, but solid copper chloride is not. (c) SiO2 melts at a very high temperature, while CO2 is a ...
... Using principles of chemical bonding and/or intermolecular forces, explain each of the following. (a) Xenon has a higher boiling point than neon has. (b) Solid copper is an excellent conductor of electricity, but solid copper chloride is not. (c) SiO2 melts at a very high temperature, while CO2 is a ...
1 2016-17 Honors Chemistry Review for the Final Exam Each unit
... g) The molecule is known to be 518 times more soluble in carbon tetrachloride than water. Does this support or conflict with the assumption about the shape of the molecule from “f” above? Explain your statement by clearly discussing the involved chemicals. What does this indicate about the type of ...
... g) The molecule is known to be 518 times more soluble in carbon tetrachloride than water. Does this support or conflict with the assumption about the shape of the molecule from “f” above? Explain your statement by clearly discussing the involved chemicals. What does this indicate about the type of ...
SampleTest3
... D) more than one response is correct ____11. Which of the following is an exothermic process? A) Sublimation [(s) to (g)] B) melting C) evaporation D) condensation ____12. The vapor pressure of a liquid A) decreases with increasing temperature B) is independent of temperature C) is equal to one atmo ...
... D) more than one response is correct ____11. Which of the following is an exothermic process? A) Sublimation [(s) to (g)] B) melting C) evaporation D) condensation ____12. The vapor pressure of a liquid A) decreases with increasing temperature B) is independent of temperature C) is equal to one atmo ...
Intro to Chem
... Color, shape, melting point, boiling point, density, strength, hardness, ability to conduct electricity, etc. Melting point – temperature and pressure at which a solid becomes a liquid Boiling point – temperature at which a liquid becomes a gas. Density – ratio of the mass of a substance to th ...
... Color, shape, melting point, boiling point, density, strength, hardness, ability to conduct electricity, etc. Melting point – temperature and pressure at which a solid becomes a liquid Boiling point – temperature at which a liquid becomes a gas. Density – ratio of the mass of a substance to th ...
solutions - Scarsdale Public Schools
... a) calculate the molarity of the ethanol/water solution: ...
... a) calculate the molarity of the ethanol/water solution: ...
Chapter 3
... • Heterogeneous = non-uniform, contains regions with different properties than other regions ...
... • Heterogeneous = non-uniform, contains regions with different properties than other regions ...
Chemistry 2nd Semester Final Review
... 1. If a gas occupies 3.8 L at a pressure of 2.71 atm, what would the pressure be if the volume changes to 1.47 L? (Temp. and amt. of gas constant) 2. A gas has a volume of 1.49 L at a temperature of 34.75 °C. What would the volume be at 78.41 °C? (pressure & amt. of gas constant) 3. What volume is o ...
... 1. If a gas occupies 3.8 L at a pressure of 2.71 atm, what would the pressure be if the volume changes to 1.47 L? (Temp. and amt. of gas constant) 2. A gas has a volume of 1.49 L at a temperature of 34.75 °C. What would the volume be at 78.41 °C? (pressure & amt. of gas constant) 3. What volume is o ...
A Study of Matter
... Phase changes • Boiling point- liquid turns to a gas (water to water vapor) • Condensation- where a gas turns to a liquid (the sweating on a glass that is colder than it’s environment) • Sublimation point- temperature at which a solid changes directly to a gas without first changing into a liquid. ...
... Phase changes • Boiling point- liquid turns to a gas (water to water vapor) • Condensation- where a gas turns to a liquid (the sweating on a glass that is colder than it’s environment) • Sublimation point- temperature at which a solid changes directly to a gas without first changing into a liquid. ...
Chem 1A Final Exam – Fall 2005
... of a 0.250 M solution of ammonium fluoride. (Assume you have an analytical balance and 2.0000L, 1.0000L, 500.00mL, and 250.00mL volumetric flasks.) Describe in detail with the correct amounts how you would make this solution: a) ...
... of a 0.250 M solution of ammonium fluoride. (Assume you have an analytical balance and 2.0000L, 1.0000L, 500.00mL, and 250.00mL volumetric flasks.) Describe in detail with the correct amounts how you would make this solution: a) ...
Participation #1 - Alan`s Chemistry Page
... 4C3H5N3O9(l) → 6N2(g) + 12CO2(g) + 10H2O(g) + O2(g) a. Calculate the molar masses for nitroglycerin, carbon dioxide, and water. ...
... 4C3H5N3O9(l) → 6N2(g) + 12CO2(g) + 10H2O(g) + O2(g) a. Calculate the molar masses for nitroglycerin, carbon dioxide, and water. ...
Physical Science
... shape, or state of matter • A Substance does not change identity when it undergoes a physical change ...
... shape, or state of matter • A Substance does not change identity when it undergoes a physical change ...