carboxylic acids - La Salle University
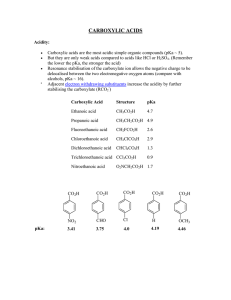
... Carboxylic acids are the most acidic simple organic compounds (pKa ~ 5). But they are only weak acids compared to acids like HCl or H2SO4. (Remember the lower the pKa, the stronger the acid) Resonance stabilisation of the carboxylate ion allows the negative charge to be delocalised between the two e ...
... Carboxylic acids are the most acidic simple organic compounds (pKa ~ 5). But they are only weak acids compared to acids like HCl or H2SO4. (Remember the lower the pKa, the stronger the acid) Resonance stabilisation of the carboxylate ion allows the negative charge to be delocalised between the two e ...
Compounds
... different types of elements that make up a compound. Sugar has the chemical formula C6H12O6. Using the periodic table it is clear that sugar is made up of the elements carbon (C), hydrogen (H), and oxygen (O). ...
... different types of elements that make up a compound. Sugar has the chemical formula C6H12O6. Using the periodic table it is clear that sugar is made up of the elements carbon (C), hydrogen (H), and oxygen (O). ...
Chemistry - NTU.edu - Nanyang Technological University
... Arenes (exemplified by benzene and methylbenzene) (a) Influence of delocalised electrons on structure and properties (b) Substitution reactions with electrophiles (c) Oxidation of side-chain ...
... Arenes (exemplified by benzene and methylbenzene) (a) Influence of delocalised electrons on structure and properties (b) Substitution reactions with electrophiles (c) Oxidation of side-chain ...
Final - Courses
... But a newer body of research says we should look instead at the molecules before deprotonation for the real explanation of the difference in acidity between carboxylic acids and alcohols. What might this explanation be, and which one do you think is correct? ...
... But a newer body of research says we should look instead at the molecules before deprotonation for the real explanation of the difference in acidity between carboxylic acids and alcohols. What might this explanation be, and which one do you think is correct? ...
Derivatives of Carboxylic Acids
... • Salicylic acid is a bifunctional molecule (acid and phenol) which is itself a disinfectant. • Aspirin comes from the willow bark which was used in the Middle Ages by Jesuit missionaries. ...
... • Salicylic acid is a bifunctional molecule (acid and phenol) which is itself a disinfectant. • Aspirin comes from the willow bark which was used in the Middle Ages by Jesuit missionaries. ...
biochemistry Notes
... 3)____________________ - structural polysaccharide in plants. (Straight 1 – 4 beta glycosidic bonds). : Carbon 6 is alternately up then down ...
... 3)____________________ - structural polysaccharide in plants. (Straight 1 – 4 beta glycosidic bonds). : Carbon 6 is alternately up then down ...
CHEM 122: Introduction to Organic Chemistry Chapter 5: Alcohols
... 20. Draw structural formulas and write common names for the six isomeric ethers with the molecular formula C5H12O. 21. 1,4-Butanediol, hexane, and 1-pentanol have similar molecular weights. Their boiling points, arranged from lowest to highest, are 69oC, 138oC, and 230oC. Which compound has which bo ...
... 20. Draw structural formulas and write common names for the six isomeric ethers with the molecular formula C5H12O. 21. 1,4-Butanediol, hexane, and 1-pentanol have similar molecular weights. Their boiling points, arranged from lowest to highest, are 69oC, 138oC, and 230oC. Which compound has which bo ...
Palmitic Acid 98% - McKinley Resources, Inc.
... Palmitic acid is mainly used to produce soaps, cosmetics, and release agents. These applications utilize sodium palmitate, which is commonly obtained by saponification of palm oil. Because it is inexpensive and adds texture to processed foods (convenience food), palmitic acid and its sodium salt fin ...
... Palmitic acid is mainly used to produce soaps, cosmetics, and release agents. These applications utilize sodium palmitate, which is commonly obtained by saponification of palm oil. Because it is inexpensive and adds texture to processed foods (convenience food), palmitic acid and its sodium salt fin ...
Organic syntheses HSCP
... Lectures on the synthesis of given types of molecules alternate with strategy lectures in which the methods just learnt are placed in a wider context. The synthesis lectures cover many ways of making each type of molecule starting with simple aromatic and aliphatic compounds with one functional grou ...
... Lectures on the synthesis of given types of molecules alternate with strategy lectures in which the methods just learnt are placed in a wider context. The synthesis lectures cover many ways of making each type of molecule starting with simple aromatic and aliphatic compounds with one functional grou ...
notes fill in File
... The lower the number, the more _________ pH 7 = base The higher the number, the more _________ An indicator is used to determine pH (litmus paper/phenolphthalein…. Other indicators are pH paper, methyl orange, bromothymol blue, red cabbage juice, and tea The most accurate measurements are done wit ...
... The lower the number, the more _________ pH 7 = base The higher the number, the more _________ An indicator is used to determine pH (litmus paper/phenolphthalein…. Other indicators are pH paper, methyl orange, bromothymol blue, red cabbage juice, and tea The most accurate measurements are done wit ...
Slide 1
... • In these polymers, two different functional groups are required and for each new bond between the monomer units (shown coloured below), a small molecule (often water) is produced. • Each monomer must also have two functional groups. • This can involve two different functional groups on the same mo ...
... • In these polymers, two different functional groups are required and for each new bond between the monomer units (shown coloured below), a small molecule (often water) is produced. • Each monomer must also have two functional groups. • This can involve two different functional groups on the same mo ...
Ch 1 Hydrocarbons
... The mechanisms can be described in terms of electron shifts, Radical substitution vice versa of alkanes. The physical properties will be explained in terms of the intermolecular forces. ...
... The mechanisms can be described in terms of electron shifts, Radical substitution vice versa of alkanes. The physical properties will be explained in terms of the intermolecular forces. ...
Lecture 14
... i not chiral hi l as it i has h a plane l off symmetry, but the two primary OH groups are enantiotopic. If one of them is changed—by esterification, for example—the molecule becomes chiral. Natural glycerol phosphate is such an es suc ester e andd it iss op optically c y active. c ve. ...
... i not chiral hi l as it i has h a plane l off symmetry, but the two primary OH groups are enantiotopic. If one of them is changed—by esterification, for example—the molecule becomes chiral. Natural glycerol phosphate is such an es suc ester e andd it iss op optically c y active. c ve. ...
Esters - Phillips Scientific Methods
... Respiration (oxidation of glucose), as ATP ADP to start the process, and the phosphate bonds ...
... Respiration (oxidation of glucose), as ATP ADP to start the process, and the phosphate bonds ...
Chapter #21 Notes
... Insert position numbers. Place the # of the OH before the parent name. Punctuate the name. ...
... Insert position numbers. Place the # of the OH before the parent name. Punctuate the name. ...
Document
... To provide the students with an understanding of the behavior of organic compounds, the interaction of the major functional groups in multifunctional compounds. In addition to that, diferent steps of some drugs synthesis including their mechanism of reactions will be discussed. Course Description: T ...
... To provide the students with an understanding of the behavior of organic compounds, the interaction of the major functional groups in multifunctional compounds. In addition to that, diferent steps of some drugs synthesis including their mechanism of reactions will be discussed. Course Description: T ...
Organic Chemistry
... Primary (1o) alcohols have hydroxyl (-OH) group at an end carbon Secondary (2o) alcohols have -OH on carbon in the middle of a chain Tertiary (3o) alcohols have -OH on a carbon that has only other carbons attached to it (no hydrogens) Compounds with more than one –OH are called “glycols” ...
... Primary (1o) alcohols have hydroxyl (-OH) group at an end carbon Secondary (2o) alcohols have -OH on carbon in the middle of a chain Tertiary (3o) alcohols have -OH on a carbon that has only other carbons attached to it (no hydrogens) Compounds with more than one –OH are called “glycols” ...
Toxic Chemicals
... Sample. (Normally we give you a pure sample), but if not You need to make sure that the sample is pure otherwise you learn nothing definitive from any test carried out on an impure compound. ...
... Sample. (Normally we give you a pure sample), but if not You need to make sure that the sample is pure otherwise you learn nothing definitive from any test carried out on an impure compound. ...
Unit C
... • compile and organize data to compare the properties of structural isomers; e.g., pairs of hydrocarbon isomers and primary, secondary and tertiary alcohols • interpret the results of a test to distinguish between a saturated and an unsaturated aliphatic, using aqueous bromine or potassium permangan ...
... • compile and organize data to compare the properties of structural isomers; e.g., pairs of hydrocarbon isomers and primary, secondary and tertiary alcohols • interpret the results of a test to distinguish between a saturated and an unsaturated aliphatic, using aqueous bromine or potassium permangan ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.























