Nugget
... carborane counterion. The optimization of the synthetic conditions for the ruthenium carborane complexes leads us in search of the new protonating agents. In addition to the ether and toluene based protonation agents ([H Ln]+ [carborane]-, where L is a solvent of choice ), we completed per-methylati ...
... carborane counterion. The optimization of the synthetic conditions for the ruthenium carborane complexes leads us in search of the new protonating agents. In addition to the ether and toluene based protonation agents ([H Ln]+ [carborane]-, where L is a solvent of choice ), we completed per-methylati ...
Document
... • HYDROLYSIS Reaction of Alkyl Hydrogen Sulfate • Simply Heat the Sulfate in Water • Net Reaction is Markovnikov Addition of H2O to Alkene • Used in One Industrial Ethanol Making Process ...
... • HYDROLYSIS Reaction of Alkyl Hydrogen Sulfate • Simply Heat the Sulfate in Water • Net Reaction is Markovnikov Addition of H2O to Alkene • Used in One Industrial Ethanol Making Process ...
A Direct Access to 3-(2-Oxoalkyl)indoles via
... dropwise. The mixture was then stirred at 25 °C according to the time indicated in Table 1. A variety of acyl chlorides were used successfully in this AlCl3-mediated C-C bond-forming reaction, and the yields of the isolated products (3) after purifying by column chromatography were found to be moder ...
... dropwise. The mixture was then stirred at 25 °C according to the time indicated in Table 1. A variety of acyl chlorides were used successfully in this AlCl3-mediated C-C bond-forming reaction, and the yields of the isolated products (3) after purifying by column chromatography were found to be moder ...
EXPERIMENT 5 (Organic Chemistry II) Pahlavan/Cherif
... Purpose - The purpose of this lab is to produce cyclohexene through the acid catalyzed elimination of water from cyclohexanol (dehydration). ...
... Purpose - The purpose of this lab is to produce cyclohexene through the acid catalyzed elimination of water from cyclohexanol (dehydration). ...
Dehydration of Alcohols - Dehydration of Cyclohexanol
... Purpose - The purpose of this lab is to produce cyclohexene through the acid catalyzed elimination of water from cyclohexanol (dehydration). ...
... Purpose - The purpose of this lab is to produce cyclohexene through the acid catalyzed elimination of water from cyclohexanol (dehydration). ...
Addition/elimination under acidic conditions
... Nucleophilic acyl substitutions can occur with weak nucleophiles under basic conditions, but only if the carboxylic acid derivative is very reactive Nulceophilic acyl substitutions can occur with weak nucleophiles under acidic conditions Carboxylic acids can be esterified with alcohols under a ...
... Nucleophilic acyl substitutions can occur with weak nucleophiles under basic conditions, but only if the carboxylic acid derivative is very reactive Nulceophilic acyl substitutions can occur with weak nucleophiles under acidic conditions Carboxylic acids can be esterified with alcohols under a ...
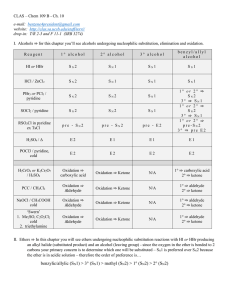
chapter 8 lecture
... • Two different starting materials can be used—a vicinal dihalide or a geminal dihalide. ...
... • Two different starting materials can be used—a vicinal dihalide or a geminal dihalide. ...
Instructor notes
... methane to methanol was reported by Shilov in 1972. A large portion of the research in “Shilov Chemistry” has focused on determining the mechanism of the original Shilov system. Shilov proposed a reasonable mechanism just a few years after the discovery of this system. The first step of this cycle i ...
... methane to methanol was reported by Shilov in 1972. A large portion of the research in “Shilov Chemistry” has focused on determining the mechanism of the original Shilov system. Shilov proposed a reasonable mechanism just a few years after the discovery of this system. The first step of this cycle i ...
Chapter 10 - UCSB CLAS
... undergoing Hoffmann (aka anti-Zaitsev) elimination reactions (only E2)– there are four carbons bonded to the nitrogen and therefore there can be up to twelve β-carbons to choose from – however in an anti-Zaitsev elimination the order of preference is benzylic/allylic > 1° > 2° > 3° VII. Sulfur funct ...
... undergoing Hoffmann (aka anti-Zaitsev) elimination reactions (only E2)– there are four carbons bonded to the nitrogen and therefore there can be up to twelve β-carbons to choose from – however in an anti-Zaitsev elimination the order of preference is benzylic/allylic > 1° > 2° > 3° VII. Sulfur funct ...
Novel amine-catalysed hydroalkoxylation reactions of
... The importance of b-hydroxycarbonyl compounds and their protected alkoxy analogues in natural product chemistry1 and organic synthesis in general2 is difficult to overstate. While the former class of compounds is readily prepared via (inter alia) the aldol reaction, the direct synthesis of b-alkoxyc ...
... The importance of b-hydroxycarbonyl compounds and their protected alkoxy analogues in natural product chemistry1 and organic synthesis in general2 is difficult to overstate. While the former class of compounds is readily prepared via (inter alia) the aldol reaction, the direct synthesis of b-alkoxyc ...
... Green Technology has been mentioned in the past few years due to the environmental problems are still increasing as a result of the environmental care products demand increased. The biological processes have thus been developed for any manufacturing steps. Alkyl glycosides are classified as a non-io ...
Nucleophilic Substitution Swapping
... The ability of solvents to stabilize ions through solvation is directly associated with their polarity. Polar solvents, such as water, can stabilize the ions 3X more than alcohol (see chart) Methanol can stabilize ions through solvation, but water is best at controlling ions. The :OH- is a super st ...
... The ability of solvents to stabilize ions through solvation is directly associated with their polarity. Polar solvents, such as water, can stabilize the ions 3X more than alcohol (see chart) Methanol can stabilize ions through solvation, but water is best at controlling ions. The :OH- is a super st ...
haloalkanes (halogenoalkanes)
... This form of nucleophilic substitution discussed so far is known as SN2; it is a bimolecular process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 ...
... This form of nucleophilic substitution discussed so far is known as SN2; it is a bimolecular process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 ...
The Formation of 2,2,4-Trimethyl-2,3-dihydro-1H-1,5
... When 0.02 mol of o-phenylenediamine were heated under reflux (80 °C) with ethanol (10 mL) and acetone (5 mL) for 8 h, the product obtained was 2,2,4-trimethyl-2,3-dihydro-1H-1,5-benzodiazopine which confirmed that the cyclization occurred without the involvement of isophthalic acid. The synthesized ...
... When 0.02 mol of o-phenylenediamine were heated under reflux (80 °C) with ethanol (10 mL) and acetone (5 mL) for 8 h, the product obtained was 2,2,4-trimethyl-2,3-dihydro-1H-1,5-benzodiazopine which confirmed that the cyclization occurred without the involvement of isophthalic acid. The synthesized ...
Summer Scholar Report
... Originally, reactions were carried out in diethyl ether since the solvent could be easily removed, but under several different conditions our yield remained low. It became clear that the sodium hydride was not sufficiently soluble in diethyl ether. Replacing diethyl ether with tetrahydrofuran (THF) ...
... Originally, reactions were carried out in diethyl ether since the solvent could be easily removed, but under several different conditions our yield remained low. It became clear that the sodium hydride was not sufficiently soluble in diethyl ether. Replacing diethyl ether with tetrahydrofuran (THF) ...
chemistry- sch4u - final exam
... 75. Barbituric acid, (H-Bar), was discovered by Adolph von Baeyer and named after a friend Barbara. It is the parent compound of a widely used sleeping drug, the barbituates. It is a weak acid with a Ka of 9.7 x 10-5. What is the [H+], the pH, and the [OH-] of a 0.05 mol/L solution of H-Bar? (6) 76. ...
... 75. Barbituric acid, (H-Bar), was discovered by Adolph von Baeyer and named after a friend Barbara. It is the parent compound of a widely used sleeping drug, the barbituates. It is a weak acid with a Ka of 9.7 x 10-5. What is the [H+], the pH, and the [OH-] of a 0.05 mol/L solution of H-Bar? (6) 76. ...
Chapter 1 Chemical Bonding and Chemical Structure
... Three Mechanistic Steps in EAS 1. Generation of an electrophile 2. Nucleophilic reaction of the p electrons of the aromatic ring with the electrophile ...
... Three Mechanistic Steps in EAS 1. Generation of an electrophile 2. Nucleophilic reaction of the p electrons of the aromatic ring with the electrophile ...
Chapter 8 Alkenes and Alkynes II: Addition Reactions Alkenes are
... Product with the more stable carbocation intermediate predominates Transition state for the rate determining step (first step) resembles a carbocation and is stabilized by factors which stabilize carbocations ...
... Product with the more stable carbocation intermediate predominates Transition state for the rate determining step (first step) resembles a carbocation and is stabilized by factors which stabilize carbocations ...
Reductive etherification of substituted cyclohexanones with
... acid-catalysed conversion of the ketones to (hemi)acetals and consecutive Meerwein–Ponndorf–Verley (MPV)-type hydride transfer to yield ethers. Until now two catalysts were always required to achieve this, i.e. a strong acid for the acetalisation and a transition metal for the reduction step.7,8 Var ...
... acid-catalysed conversion of the ketones to (hemi)acetals and consecutive Meerwein–Ponndorf–Verley (MPV)-type hydride transfer to yield ethers. Until now two catalysts were always required to achieve this, i.e. a strong acid for the acetalisation and a transition metal for the reduction step.7,8 Var ...
CBS Reduction
... • They used BH3 , (85% 2-MeTHF, 15% THF) and oxazaborolidine for reduction. • Under such reaction conditions, the reaction was complete in 10 minutes and alcohol was produced with 95% yield and a 91 : 9 enantiomeric ratio (highly enantioselectivety). ...
... • They used BH3 , (85% 2-MeTHF, 15% THF) and oxazaborolidine for reduction. • Under such reaction conditions, the reaction was complete in 10 minutes and alcohol was produced with 95% yield and a 91 : 9 enantiomeric ratio (highly enantioselectivety). ...
Alkyl and Aryl Halides
... present in high concentrations favor SN2 reactions. • Weak nucleophiles, such as H2O and ROH favor SN1 reactions by decreasing the rate of any competing SN2 reaction. • Let us compare the substitution products formed when the 2° alkyl halide A is treated with either the strong nucleophile HO¯ or the ...
... present in high concentrations favor SN2 reactions. • Weak nucleophiles, such as H2O and ROH favor SN1 reactions by decreasing the rate of any competing SN2 reaction. • Let us compare the substitution products formed when the 2° alkyl halide A is treated with either the strong nucleophile HO¯ or the ...
CN>Chapter 22CT>Carbonyl Alpha
... Synthesis of carboxylic acids using malonic ester via the decarboxylation of malonic acid The malonic ester synthesis converts an alkyl halide into a ...
... Synthesis of carboxylic acids using malonic ester via the decarboxylation of malonic acid The malonic ester synthesis converts an alkyl halide into a ...
Background Information
... stable carbocation intermediates will undergo the reaction. The acid catalyst activates the OH group of the alcohol by protonating the oxygen atom. The C-OH2+ bond breaks to generate the carbocation, which in turn reacts with the chloride ion (nucleophile) to generate an alkyl halide product. A gene ...
... stable carbocation intermediates will undergo the reaction. The acid catalyst activates the OH group of the alcohol by protonating the oxygen atom. The C-OH2+ bond breaks to generate the carbocation, which in turn reacts with the chloride ion (nucleophile) to generate an alkyl halide product. A gene ...
chemistry 232 elementary organic chemistry ii
... Acid-Base Protonation/Deprotonation Reactions (Ch. 7 & 10) Protonation/Deprotonation of Alcohols Deprotonation of Alkynes Acid-Catalyzed Rearrangements (Ch. 9 & 10) via SN1 Reaction Pathway (step-wise) The Pinacol Rearrangement Nucleophilic Addition to Carbonyl Compounds (Ch. 15, 16, & 17) Organomet ...
... Acid-Base Protonation/Deprotonation Reactions (Ch. 7 & 10) Protonation/Deprotonation of Alcohols Deprotonation of Alkynes Acid-Catalyzed Rearrangements (Ch. 9 & 10) via SN1 Reaction Pathway (step-wise) The Pinacol Rearrangement Nucleophilic Addition to Carbonyl Compounds (Ch. 15, 16, & 17) Organomet ...
Vinylcyclopropane rearrangement

The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring.Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.Due to its ability to form cyclopentene rings the vinylcyclopropane rearrangement has served several times as a key reaction in complex natural product synthesis.