Recall
... Because in basic media (acid destroys Gilman) oxygen of oxirane can not be protonated. Less hindered carbon of oxirane is attacked. ...
... Because in basic media (acid destroys Gilman) oxygen of oxirane can not be protonated. Less hindered carbon of oxirane is attacked. ...
Electrophilic Additions: Alkenes Addition of Hydrogen Halides
... In a regioselective reaction, one constitutional isomer is the major or the only product. I: early transition state (Like reactants) ...
... In a regioselective reaction, one constitutional isomer is the major or the only product. I: early transition state (Like reactants) ...
- University at Albany
... Racemization - not always exactly 50/50. Carbocation can be attacked from the top or bottom face giving both enantiomers. Steric hindrance gives attack at one side preferentially Longer-lived carbocations give more racemization, shorter-lived give more inversion ...
... Racemization - not always exactly 50/50. Carbocation can be attacked from the top or bottom face giving both enantiomers. Steric hindrance gives attack at one side preferentially Longer-lived carbocations give more racemization, shorter-lived give more inversion ...
ALDOL CONDENSATION
... The reactions between a ketone and a carbonyl compound lacking an alpha‐Hydrogen(Cross Aldol condensation) is called Claisen‐Schmidt condensation. These reactions are named after two of its pioneering investigators Rainer Ludwig Claisen and J. G. Schmidt, who independently published on this to ...
... The reactions between a ketone and a carbonyl compound lacking an alpha‐Hydrogen(Cross Aldol condensation) is called Claisen‐Schmidt condensation. These reactions are named after two of its pioneering investigators Rainer Ludwig Claisen and J. G. Schmidt, who independently published on this to ...
Organic Chemistry Fifth Edition
... mechanism must equal the overall reaction equation. The reaction is a substitution reaction in which the nucleophile chloride takes the place of the OH. Thus, it is known as an SN reaction. ...
... mechanism must equal the overall reaction equation. The reaction is a substitution reaction in which the nucleophile chloride takes the place of the OH. Thus, it is known as an SN reaction. ...
Exam 2 Review A
... mechanism [with the exception of 8c] to explain how these reactions work, starting from: a. A base-induced alpha-elmination of chloroform. b. Diazomethane. c. The Simmons-Smith reagent. 9. Be able to explain how OsO4 or KMnO4 can be used with variable success to syndihydroxylate alkenes, using an ar ...
... mechanism [with the exception of 8c] to explain how these reactions work, starting from: a. A base-induced alpha-elmination of chloroform. b. Diazomethane. c. The Simmons-Smith reagent. 9. Be able to explain how OsO4 or KMnO4 can be used with variable success to syndihydroxylate alkenes, using an ar ...
Chapter 9 Alcohols, Ethers, and Epoxides
... • The ring is then numbered in a clockwise or counterclockwise fashion to give the next substituent the lowest number. Figure 9.2 Examples: Naming cyclic alcohols ...
... • The ring is then numbered in a clockwise or counterclockwise fashion to give the next substituent the lowest number. Figure 9.2 Examples: Naming cyclic alcohols ...
Chapter16McMurryPPP
... This forms a cationic addition intermediate from benzene and a bromine cation The intermediate is not aromatic and therefore high in energy (see Figure 16.2) ...
... This forms a cationic addition intermediate from benzene and a bromine cation The intermediate is not aromatic and therefore high in energy (see Figure 16.2) ...
Chapter 20. Aldehydes and Ketones
... chemical yield would be high, our dedicated student prepared one mole of the Grignard reagent, added two moles of benzaldehyde, and, after working up the reaction, was delighted to obtain a good yield of a crystalline product. Unfortunately, the product that had been formed was benzophenone! On clos ...
... chemical yield would be high, our dedicated student prepared one mole of the Grignard reagent, added two moles of benzaldehyde, and, after working up the reaction, was delighted to obtain a good yield of a crystalline product. Unfortunately, the product that had been formed was benzophenone! On clos ...
The Fischer Indole Synthesis
... derivative. Several thousand indole derivatives appear annually in chemical literature.3 The Fischer indole synthesis is the most widely used and versatile method for indole synthesis. Reaction Mechanism: The Fischer indole synthesis converts ayrlhydrazones of aldehydes or ketones into indoles in th ...
... derivative. Several thousand indole derivatives appear annually in chemical literature.3 The Fischer indole synthesis is the most widely used and versatile method for indole synthesis. Reaction Mechanism: The Fischer indole synthesis converts ayrlhydrazones of aldehydes or ketones into indoles in th ...
Document
... • In order for ethers to undergo substitution or elimination reactions, their poor leaving group must first be converted into a good leaving group by reaction with strong acids such as HBr and HI. HBr and HI are strong acids that are also sources of good nucleophiles (Br¯ and I¯ respectively). • Whe ...
... • In order for ethers to undergo substitution or elimination reactions, their poor leaving group must first be converted into a good leaving group by reaction with strong acids such as HBr and HI. HBr and HI are strong acids that are also sources of good nucleophiles (Br¯ and I¯ respectively). • Whe ...
synthesis in industry
... give very low yields of the desired 2-alkyl-3-chloronaphthoquinones. Dialkylation and reduction of the quinone are major complications. Mild alkylating agents such as tetraalkyltins do not react with the dichloro compound unassisted, but we found that the alkylation can be catalyzed. For example, th ...
... give very low yields of the desired 2-alkyl-3-chloronaphthoquinones. Dialkylation and reduction of the quinone are major complications. Mild alkylating agents such as tetraalkyltins do not react with the dichloro compound unassisted, but we found that the alkylation can be catalyzed. For example, th ...
14_chapter 8
... Under the optimised condition, various benzyl bromides were reacted with 20% sodium nitrate solution at a temperature of 120 oC to obtain the corresponding benzaldehydes (Table 8.1). In the present scheme, we are not using any of the costly oxidising agents such as manganese dioxide, peracid, period ...
... Under the optimised condition, various benzyl bromides were reacted with 20% sodium nitrate solution at a temperature of 120 oC to obtain the corresponding benzaldehydes (Table 8.1). In the present scheme, we are not using any of the costly oxidising agents such as manganese dioxide, peracid, period ...
22.4: Acidity of Phenols.
... Electron-withdrawing substituents make a phenol more acidic by stabilizing the phenoxide ion through delocalization of the ...
... Electron-withdrawing substituents make a phenol more acidic by stabilizing the phenoxide ion through delocalization of the ...
A Model for Catalytically Active Zinc(I1) Ion in Liver
... nucleophile to carbonyls (aldehydes and esters),I0 as a bifunctional nucleophile to phosphates," and as a base toward amidesI3 and sulfonamide^.'^ We now turn our attention to the similar pK, values for Zn"-OH2 in ADH5 and 1. In this report we show that L,-Zn"-OH- is indeed a very good catalyst for ...
... nucleophile to carbonyls (aldehydes and esters),I0 as a bifunctional nucleophile to phosphates," and as a base toward amidesI3 and sulfonamide^.'^ We now turn our attention to the similar pK, values for Zn"-OH2 in ADH5 and 1. In this report we show that L,-Zn"-OH- is indeed a very good catalyst for ...
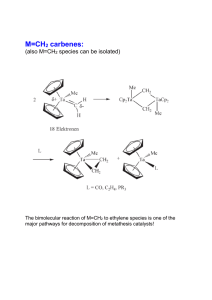
Steric protection of alkylidene is not needed:
... The bimolecular reaction of M=CH2 to ethylene species is one of the major pathways for decomposition of metathesis catalysts! ...
... The bimolecular reaction of M=CH2 to ethylene species is one of the major pathways for decomposition of metathesis catalysts! ...
Enantioselective Synthesis of Cyclic Ethers through a Vanadium
... spite of the chiral ligand employed, the epoxidation/ringopening reaction gave racemic 2,5-cis-THF rings as the major product. In accord with this report, our own attempts to introduce enantioselectivity in this reaction by resolution of bishomoallylic alcohols by catalytic epoxidation[4] resulted i ...
... spite of the chiral ligand employed, the epoxidation/ringopening reaction gave racemic 2,5-cis-THF rings as the major product. In accord with this report, our own attempts to introduce enantioselectivity in this reaction by resolution of bishomoallylic alcohols by catalytic epoxidation[4] resulted i ...
Ch 23 Carbonyl Condensations
... - This reaction also begins with an alkoxide base (NaOEt) removing an H to create an enolate. - This reaction is different from aldol and Claisen condensations in that the enolate (donor or Nu-) adds to the C of a conjugated enone (acceptor or E+). This is identical to the conjugated enone addit ...
... - This reaction also begins with an alkoxide base (NaOEt) removing an H to create an enolate. - This reaction is different from aldol and Claisen condensations in that the enolate (donor or Nu-) adds to the C of a conjugated enone (acceptor or E+). This is identical to the conjugated enone addit ...
Synopsis
... products with the same configuration at carbon. An efficient route to αhydroxy-β-amino acid derivatives AHDA and AHPBA was developed using a common advanced intermediate. The methodology provides aminoalcohol derivatives with a Cbz group on nitrogen that can be deprotected under mild reaction condit ...
... products with the same configuration at carbon. An efficient route to αhydroxy-β-amino acid derivatives AHDA and AHPBA was developed using a common advanced intermediate. The methodology provides aminoalcohol derivatives with a Cbz group on nitrogen that can be deprotected under mild reaction condit ...
Synthesis of Four Diastereomeric 3,5-Dialkoxy-2,4
... treatment of the dimesylate 24 with 2.4 equiv of methylmagnesium bromide in THF at 0 °C for 10 min afforded the desired diol 25 in 92% yield.11 ...
... treatment of the dimesylate 24 with 2.4 equiv of methylmagnesium bromide in THF at 0 °C for 10 min afforded the desired diol 25 in 92% yield.11 ...
thiols and sulfides.
... The intramolecular Williamson synthesis is stereospecific Since the Williamson synthesis is a SN2 substitution reaction, an inversion of configuration occurs at the carbon bearing the leaving group. The leaving group must be on the opposite side of the molecule from the attacking nucleophile in ord ...
... The intramolecular Williamson synthesis is stereospecific Since the Williamson synthesis is a SN2 substitution reaction, an inversion of configuration occurs at the carbon bearing the leaving group. The leaving group must be on the opposite side of the molecule from the attacking nucleophile in ord ...
Synthesis of (−)-Epibatidine - David A. Evans
... activated acyl oxazolidinone.6 Furthermore, previous contributions by Ghosez and co-workers have established that Diels-Alder reactions utilizing 2-azadienes such as 4 are highly exo-selective.5 The union of these two control elements would afford an appropriately functionalized 2-azabicyclo[2.2.2]o ...
... activated acyl oxazolidinone.6 Furthermore, previous contributions by Ghosez and co-workers have established that Diels-Alder reactions utilizing 2-azadienes such as 4 are highly exo-selective.5 The union of these two control elements would afford an appropriately functionalized 2-azabicyclo[2.2.2]o ...
Studies of Carbon-Sulfur Bond Cleavage by Homogeneous
... carbon-sulfur bond breaking step. The structures of the intermediates involved were elucidated and the kinetic and thermodynamic parameters that control the reactivity and selectivity were determined. New complexes were found that not only break C-S bonds, but also do further chemistry resulting in ...
... carbon-sulfur bond breaking step. The structures of the intermediates involved were elucidated and the kinetic and thermodynamic parameters that control the reactivity and selectivity were determined. New complexes were found that not only break C-S bonds, but also do further chemistry resulting in ...
temperature and diffusion
... energy (Ea) that is required to convert the substrates to products. In aqueous solutions Ea is an intrinsic feature of the enzyme itself (Jenta et al. 1997). However, immobilizing the enzyme, diffusion may become rate limiting and the reaction may switch from kinetic to diffusion controlled resultin ...
... energy (Ea) that is required to convert the substrates to products. In aqueous solutions Ea is an intrinsic feature of the enzyme itself (Jenta et al. 1997). However, immobilizing the enzyme, diffusion may become rate limiting and the reaction may switch from kinetic to diffusion controlled resultin ...
BHR - A Brief History - Process Intensification Network
... Kenics static mixers for low pressure drop Shell and Tube construction ...
... Kenics static mixers for low pressure drop Shell and Tube construction ...
Vinylcyclopropane rearrangement

The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring.Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.Due to its ability to form cyclopentene rings the vinylcyclopropane rearrangement has served several times as a key reaction in complex natural product synthesis.