Results
... (H2O)2 (806, 839), (H2O)3 (871, 888), valamint CH2CH2 (681, 681), CH3CHCH2 (752, 748), (CH3)2CCH2 (796, 806) Solvatation: water cluster models. In these reactions the proton affinity of the medium is an important factor (the water in the medium does not participate in the reaction but has a catalyti ...
... (H2O)2 (806, 839), (H2O)3 (871, 888), valamint CH2CH2 (681, 681), CH3CHCH2 (752, 748), (CH3)2CCH2 (796, 806) Solvatation: water cluster models. In these reactions the proton affinity of the medium is an important factor (the water in the medium does not participate in the reaction but has a catalyti ...
Bimolecular reactions of the chromium
... ligand. This is of particular interest if the free ligand is unstable and difficult to handle under the conditions typical of synthetic chemistry. Benzyne (18-didehydrobenzene) and other arynes are very reactive intermediates3 which have to be prepared in situ during a chemical syntheeis.’ However, ...
... ligand. This is of particular interest if the free ligand is unstable and difficult to handle under the conditions typical of synthetic chemistry. Benzyne (18-didehydrobenzene) and other arynes are very reactive intermediates3 which have to be prepared in situ during a chemical syntheeis.’ However, ...
CH 908: Mass Spectrometry Lecture 3
... Chain branching clearly influences the fragmentation of this isomeric hexane. The molecular ion at m/z=86 is weaker than that for hexane itself and the M-15 ion at m/z=71 is stronger. The m/z=57 ion is almost absent (try to find a simple cleavage that gives a butyl group). An isopropyl cation (m/z=4 ...
... Chain branching clearly influences the fragmentation of this isomeric hexane. The molecular ion at m/z=86 is weaker than that for hexane itself and the M-15 ion at m/z=71 is stronger. The m/z=57 ion is almost absent (try to find a simple cleavage that gives a butyl group). An isopropyl cation (m/z=4 ...
SCI2199 - Introduction to Organic Chemistry II
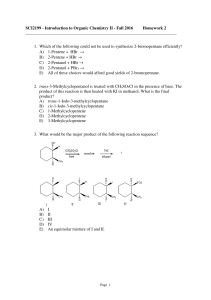
... A) an SN1-type reaction involving the protonated alcohol as the substrate. B) an SN2-type reaction involving the protonated alcohol as the substrate. C) an E1-type reaction involving the protonated alcohol as the substrate. D) an E2-type reaction involving the protonated alcohol as the substrate. E) ...
... A) an SN1-type reaction involving the protonated alcohol as the substrate. B) an SN2-type reaction involving the protonated alcohol as the substrate. C) an E1-type reaction involving the protonated alcohol as the substrate. D) an E2-type reaction involving the protonated alcohol as the substrate. E) ...
Document
... A single-step process in which both the alkyl halide and the nucleophile are involved at the transition state. Cleavage of the bond between carbon and the leaving group is assisted by formation of a bond between carbon and the nucleophile. In effect, the nucleophile “pushes off” the leaving group fr ...
... A single-step process in which both the alkyl halide and the nucleophile are involved at the transition state. Cleavage of the bond between carbon and the leaving group is assisted by formation of a bond between carbon and the nucleophile. In effect, the nucleophile “pushes off” the leaving group fr ...
reactions.html Reaction 1. Electrophilic addition of
... product (Pd, CaCO3, Pb(OAc)2, quinoline) lithium metal in ammonia for trans product ...
... product (Pd, CaCO3, Pb(OAc)2, quinoline) lithium metal in ammonia for trans product ...
Answers
... 7. Retrosynthesis: Give the structure of the alcohol(s) and the carbonyl compound necessary to make these acetals under acidic conditions. ...
... 7. Retrosynthesis: Give the structure of the alcohol(s) and the carbonyl compound necessary to make these acetals under acidic conditions. ...
TOPIC 7. ELIMINATION REACTIONS (chapter 7 and parts of
... 1. Describe mechanisms for elimination of a leaving group and adjacent proton to form a pi-bond. 2. Discuss the effect of starting material (“substrate”), leaving group, and reaction conditions on the course and outcome of a reaction. 3. Describe syntheses of alkenes and alkynes. 4. Use combinations ...
... 1. Describe mechanisms for elimination of a leaving group and adjacent proton to form a pi-bond. 2. Discuss the effect of starting material (“substrate”), leaving group, and reaction conditions on the course and outcome of a reaction. 3. Describe syntheses of alkenes and alkynes. 4. Use combinations ...
ppt
... Conclusion • Very simple strategy for engaging CO2 in C–C bond formation that does not require synthetic or biological catalysts. • The ability to deprotonate unactivated C–H bonds opens the possibility of using this approach to prepare numerous high-volume targets. ...
... Conclusion • Very simple strategy for engaging CO2 in C–C bond formation that does not require synthetic or biological catalysts. • The ability to deprotonate unactivated C–H bonds opens the possibility of using this approach to prepare numerous high-volume targets. ...
Lecture12
... optimal conditions for every new substrate Reactions are easily poisoned by molecular oxygen ...
... optimal conditions for every new substrate Reactions are easily poisoned by molecular oxygen ...
File - Dr KHALID SHADID
... (b) The magnesium portion of the Grignard reagent plays an important part in this reaction. What is its ftnction? (c) What product is formed initially? (d) What product forms when water is added? ...
... (b) The magnesium portion of the Grignard reagent plays an important part in this reaction. What is its ftnction? (c) What product is formed initially? (d) What product forms when water is added? ...
EXPERIMENT 3: Preparation and Reactivity of Alkyl Halides
... or solid phase. The method can be used to follow the course of a reaction both qualitatively and quantitatively, to separate and isolate components of a mixture, to examine purity, and to assist in the identification of compounds. The compound mixture is passed through a heated column (glass capilla ...
... or solid phase. The method can be used to follow the course of a reaction both qualitatively and quantitatively, to separate and isolate components of a mixture, to examine purity, and to assist in the identification of compounds. The compound mixture is passed through a heated column (glass capilla ...
The Aldol Condensation Preparation of 4
... Preparation of 4-Methoxybenzalacetone In this experiment 4-Methoxybenzalacetone, obtained through an aldol condensation of 4-Methoxybenzaldehyde and Acetone, will be synthesized in a one pot reaction. ...
... Preparation of 4-Methoxybenzalacetone In this experiment 4-Methoxybenzalacetone, obtained through an aldol condensation of 4-Methoxybenzaldehyde and Acetone, will be synthesized in a one pot reaction. ...
EXPERIMENT 3: The Grignard Reaction: Synthesis of
... The experiment is based on a procedure reported by J. A. Ciaccio, R. P. Bravo, A. L. Drahus, J. B. Biggins, R. V. Concepcion and D. Cabrera, Department of Chemistry, Fordham University. Thanks to Barbora Bajtos, a former 283g student, who reviewed this laboratory. General Concepts The reactions invo ...
... The experiment is based on a procedure reported by J. A. Ciaccio, R. P. Bravo, A. L. Drahus, J. B. Biggins, R. V. Concepcion and D. Cabrera, Department of Chemistry, Fordham University. Thanks to Barbora Bajtos, a former 283g student, who reviewed this laboratory. General Concepts The reactions invo ...
NaBH4 Reduction of Vanillin
... reacts only slowly with water and alcohols and can be used in a wide range of solvents, including water and alcohols, without consequence. It should also be noted that when using water as solvent, NaBH4 is relatively stable at pH 10 or higher. However, if a weakly acidic proton is present (i.e. a ...
... reacts only slowly with water and alcohols and can be used in a wide range of solvents, including water and alcohols, without consequence. It should also be noted that when using water as solvent, NaBH4 is relatively stable at pH 10 or higher. However, if a weakly acidic proton is present (i.e. a ...
CHEM 203 Topics Discussed on Nov. 20 Principle: protonation of
... Electrophilic character of the above reagents and facile reaction thereof with nucleophiles Principle: the above reagents rely on the nucleophilic properties of the OH group to achieve conversion of alcohols into alkyl halides Principle: only primary and secondary alcohols are sufficiently nucleophi ...
... Electrophilic character of the above reagents and facile reaction thereof with nucleophiles Principle: the above reagents rely on the nucleophilic properties of the OH group to achieve conversion of alcohols into alkyl halides Principle: only primary and secondary alcohols are sufficiently nucleophi ...
Reactions of Alkenes
... • Heat of reaction, H: the difference in energy between reactants and products – exothermic: products are lower in energy than reactants; heat is released – endothermic: products are higher in energy than reactants; heat is absorbed ...
... • Heat of reaction, H: the difference in energy between reactants and products – exothermic: products are lower in energy than reactants; heat is released – endothermic: products are higher in energy than reactants; heat is absorbed ...
Chapter 8 Alkenes and Alkynes II
... t Boron hydride adds successively to three molecules of alkene ...
... t Boron hydride adds successively to three molecules of alkene ...
A Biocatalytic Henry Reaction-The Hydroxynitrile Lyase from Hevea
... absolute configuration of the product was determined to be S,[5] which is in agreement with the known stereopreference of HbHNL in cyanohydrin reactions. Although the nitroaldol reaction has been known for more than a century,[6] stereoselective protocols started to evolve only a few decades ago. In ...
... absolute configuration of the product was determined to be S,[5] which is in agreement with the known stereopreference of HbHNL in cyanohydrin reactions. Although the nitroaldol reaction has been known for more than a century,[6] stereoselective protocols started to evolve only a few decades ago. In ...
Carolina Aguirre, Rosa Arrieta, Soledad Anjarí, Andrés Illanes
... Penicillin acylase (penicillin amidohydrolase;EC 3.5.1.11) is a moderately priced readily available enzyme (1) with remarkable versatility (2). It is currently used for the industrial production of 6-aminopenicillanic acid (6-A PA ) by hydrolysis of penicillin G (3) or V (4) and 7-aminodesacetoxycep ...
... Penicillin acylase (penicillin amidohydrolase;EC 3.5.1.11) is a moderately priced readily available enzyme (1) with remarkable versatility (2). It is currently used for the industrial production of 6-aminopenicillanic acid (6-A PA ) by hydrolysis of penicillin G (3) or V (4) and 7-aminodesacetoxycep ...
Aminoketone Rearrangements. 11. The Rearrangement of Phenyl a
... heated with methylamine a t 240°, 71% of the starting material was recovered with only a trace of basic material formed. The problem thus became the determination of the mechanism for the conversion of I I a to IIIa. The infrared spectra of the products obtained from the reaction of IIa with methyla ...
... heated with methylamine a t 240°, 71% of the starting material was recovered with only a trace of basic material formed. The problem thus became the determination of the mechanism for the conversion of I I a to IIIa. The infrared spectra of the products obtained from the reaction of IIa with methyla ...
Substitution Rxns-a-Sn2-12-quesx
... at the University of Freiburg, in Germany, in collaboration with William L. Hase's group at Texas Tech University, provide direct evidence for this mechanism in the gas phase. However, they also detected an additional, unexpected mechanism. In this new pathway, called the roundabout mechanism, chlor ...
... at the University of Freiburg, in Germany, in collaboration with William L. Hase's group at Texas Tech University, provide direct evidence for this mechanism in the gas phase. However, they also detected an additional, unexpected mechanism. In this new pathway, called the roundabout mechanism, chlor ...
Ethers, Sulfides, Epoxides - City University of New York
... as protecting groups for alcohols. Recall that the key step in forming the acetal was creating the carbocation as shown… There are other ways to create carbocations…… ...
... as protecting groups for alcohols. Recall that the key step in forming the acetal was creating the carbocation as shown… There are other ways to create carbocations…… ...
Microwave-Enhanced Sulphated Zirconia and SZ/MCM
... applications including organic synthesis, wherein chemical reactions are accelerated because of selective absorption of MW energy by polar molecules, non-polar molecules being inert to the MW dielectric loss [18]. Recently, it has been found that the use of microwave irradiation to assist organic re ...
... applications including organic synthesis, wherein chemical reactions are accelerated because of selective absorption of MW energy by polar molecules, non-polar molecules being inert to the MW dielectric loss [18]. Recently, it has been found that the use of microwave irradiation to assist organic re ...
Vinylcyclopropane rearrangement

The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring.Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.Due to its ability to form cyclopentene rings the vinylcyclopropane rearrangement has served several times as a key reaction in complex natural product synthesis.