Carbohydrates
... Except for dihydroxyacetone all of the monosaccharides depicted here contain one or more chiral carbon atoms. All of the sugars presented here are the D-enantiomeric form. When more than one chiral carbon is present in the molecule how is the D-form determined? If the configuration of the chiral car ...
... Except for dihydroxyacetone all of the monosaccharides depicted here contain one or more chiral carbon atoms. All of the sugars presented here are the D-enantiomeric form. When more than one chiral carbon is present in the molecule how is the D-form determined? If the configuration of the chiral car ...
Carbohydrates
... atoms that can be present in а monosaccharide, only monosaccharides with three to seven carbon atoms are commonly found in nature. А three-carbon monosaccharide is called а triose, and those that contain four, five, and six carbon atoms are called tetroses, pentoses, and hexoses, respectively. Monos ...
... atoms that can be present in а monosaccharide, only monosaccharides with three to seven carbon atoms are commonly found in nature. А three-carbon monosaccharide is called а triose, and those that contain four, five, and six carbon atoms are called tetroses, pentoses, and hexoses, respectively. Monos ...
Review for Ch. 3 Test Retake– complete on a separate sheet
... -Draw the isomers and stereoisomers of C2H4O. What are the similarities and differences between isomers and stereoisomers. Carbohydrates -Be able to identify alpha and beta glucose and fructose forms. -What are the different forms and functions of a polysaccharide Fats -How can you tell the differen ...
... -Draw the isomers and stereoisomers of C2H4O. What are the similarities and differences between isomers and stereoisomers. Carbohydrates -Be able to identify alpha and beta glucose and fructose forms. -What are the different forms and functions of a polysaccharide Fats -How can you tell the differen ...
Macromolecules of Life
... There!are'four'classes'of'macromolecules!(polysaccharides!or!carbohydrates,!triglycerides!or! lipids,!polypeptides!or!proteins,!and!nucleic'acids!such!as!DNA!and!RNA).!!Carbohydrates'and' lipids!are!made!of!only!carbon,!hydrogen,!and!oxygen!(CHO).!!Proteins!are!made!of!carbon,! hydrogen,!oxygen,!and ...
... There!are'four'classes'of'macromolecules!(polysaccharides!or!carbohydrates,!triglycerides!or! lipids,!polypeptides!or!proteins,!and!nucleic'acids!such!as!DNA!and!RNA).!!Carbohydrates'and' lipids!are!made!of!only!carbon,!hydrogen,!and!oxygen!(CHO).!!Proteins!are!made!of!carbon,! hydrogen,!oxygen,!and ...
KFUPM
... In juice purification also, ion exchanger method is used. the substitution of Ca2+ and Mg2+ for the alkali ions (Na+, K+) softens the thin syrup and prevents the formation of hardness scale on the evaporator coil. The larger pore ion exchangers also help in bleaching of the thin juice due to the bin ...
... In juice purification also, ion exchanger method is used. the substitution of Ca2+ and Mg2+ for the alkali ions (Na+, K+) softens the thin syrup and prevents the formation of hardness scale on the evaporator coil. The larger pore ion exchangers also help in bleaching of the thin juice due to the bin ...
Roy Ronalds Biochemistry I Fall 2003 Problem Set #3
... Problem Set #3 - Carbohydrates 1. In the monosaccharide derivatives known as sugar alcohols, the carbonyl oxygen is reduced to a hydroxyl group. For example, D-glyceraldehyde can be reduced to glycerol. However, this sugar alcohol is no longer designated D or L. Why? This is because the structure of ...
... Problem Set #3 - Carbohydrates 1. In the monosaccharide derivatives known as sugar alcohols, the carbonyl oxygen is reduced to a hydroxyl group. For example, D-glyceraldehyde can be reduced to glycerol. However, this sugar alcohol is no longer designated D or L. Why? This is because the structure of ...
File
... All carbohydrates are simply a chain of one or more sugars bonded together. • They are the body’s main source of energy. ...
... All carbohydrates are simply a chain of one or more sugars bonded together. • They are the body’s main source of energy. ...
Organic Compounds
... Organic chemistry is the study of carbon compounds. 90% of compounds contain Carbon Carbon has a unique ability to bond with itself in ...
... Organic chemistry is the study of carbon compounds. 90% of compounds contain Carbon Carbon has a unique ability to bond with itself in ...
Organic Molecule Worksheet
... 29. Name a waxy lipid covering plants. 30. Plant pigments like ___ are also ___. 31. Lipids have more ___ and ___ than they do oxygen atoms. 32. Fats are made of an alcohol called ___ and three ___ ___ chains. This is known as a ___. 33. If there are all SINGLE bonds between ___ in the fatty acid ch ...
... 29. Name a waxy lipid covering plants. 30. Plant pigments like ___ are also ___. 31. Lipids have more ___ and ___ than they do oxygen atoms. 32. Fats are made of an alcohol called ___ and three ___ ___ chains. This is known as a ___. 33. If there are all SINGLE bonds between ___ in the fatty acid ch ...
What are the functions of biomolecules
... • Monounsaturated f.a. = contain one double bond • Polyunsaturated f.a. = contain two or more double bond • Saturated f.a = only single bond (saturated with hydrogens!) • one carboxylic acid with even C number & no branching ...
... • Monounsaturated f.a. = contain one double bond • Polyunsaturated f.a. = contain two or more double bond • Saturated f.a = only single bond (saturated with hydrogens!) • one carboxylic acid with even C number & no branching ...
2-3 Carbon Compounds: Organic Biomolecules
... What does “poly” mean? – many So What is a monomer? – One piece is a monomer…. ...
... What does “poly” mean? – many So What is a monomer? – One piece is a monomer…. ...
BIOLOGY 189 Fundamentals of Life Science Fall 2002
... glycogen, but forms unbranched fibrils supported by hydrogen bonds Forms plant cell walls; major component of wood Cannot be hydrolyzed by most animals. Fiber or roughage. Requires microorganisms (cows / termites) ...
... glycogen, but forms unbranched fibrils supported by hydrogen bonds Forms plant cell walls; major component of wood Cannot be hydrolyzed by most animals. Fiber or roughage. Requires microorganisms (cows / termites) ...
Ecology
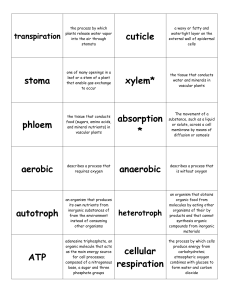
... its own nutrients from inorganic substances of from the environment instead of consuming other organisms ...
... its own nutrients from inorganic substances of from the environment instead of consuming other organisms ...
Carbohydrates - Home Economics Careers and Technology (HECT)
... cereals, corn, potatoes and legumes. Legumes are dried peas and beans. Lentils are also legumes. Your body burns starches efficiently to provide energy. Most starchy foods are also good sources of vitamins, minerals and fiber. Plant material that cannot be digested by human enzymes is called dietar ...
... cereals, corn, potatoes and legumes. Legumes are dried peas and beans. Lentils are also legumes. Your body burns starches efficiently to provide energy. Most starchy foods are also good sources of vitamins, minerals and fiber. Plant material that cannot be digested by human enzymes is called dietar ...
Macromolecules Basic Facts: Most are polymers – large molecules
... 3. Broken apart by hydrolysis reactions (break bond by adding water) ...
... 3. Broken apart by hydrolysis reactions (break bond by adding water) ...
Chapter 2-3 Carbon Compounds
... Formed from polymerization, the joining of smaller molecules into large molecules. b) Smaller molecules are called monomers and when joined together form polymers. polymers ...
... Formed from polymerization, the joining of smaller molecules into large molecules. b) Smaller molecules are called monomers and when joined together form polymers. polymers ...
Carbohydrates (cont.)
... Monomer – a simple sugar (1C:2H:1O ratio) Formula = (CH2O)n ; where n = 3-8 Common types: Glucose - - used in cells Fructose - - found in fruits Galactose - - found in milk All have same formula = C6H12O6 Isomers = same formula, different structures ...
... Monomer – a simple sugar (1C:2H:1O ratio) Formula = (CH2O)n ; where n = 3-8 Common types: Glucose - - used in cells Fructose - - found in fruits Galactose - - found in milk All have same formula = C6H12O6 Isomers = same formula, different structures ...
nucleic acid
... Since lipids are indeed, not polymers, but macromolecules made of many different subunits…they do NOT contain monomers. And since each type of lipid contains a different set of building-blocks, what you need to know is that lipids are composed of subunits known as glycerol, and fatty acids. Since th ...
... Since lipids are indeed, not polymers, but macromolecules made of many different subunits…they do NOT contain monomers. And since each type of lipid contains a different set of building-blocks, what you need to know is that lipids are composed of subunits known as glycerol, and fatty acids. Since th ...
Carbohydrate
A carbohydrate is a biological molecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen:oxygen atom ratio of 2:1 (as in water); in other words, with the empirical formula Cm(H2O)n (where m could be different from n). Some exceptions exist; for example, deoxyribose, a sugar component of DNA, has the empirical formula C5H10O4. Carbohydrates are technically hydrates of carbon; structurally it is more accurate to view them as polyhydroxy aldehydes and ketones.The term is most common in biochemistry, where it is a synonym of saccharide, a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. In general, the monosaccharides and disaccharides, which are smaller (lower molecular weight) carbohydrates, are commonly referred to as sugars. The word saccharide comes from the Greek word σάκχαρον (sákkharon), meaning ""sugar."" While the scientific nomenclature of carbohydrates is complex, the names of the monosaccharides and disaccharides very often end in the suffix -ose. For example, grape sugar is the monosaccharide glucose, cane sugar is the disaccharide sucrose and milk sugar is the disaccharide lactose (see illustration).Carbohydrates perform numerous roles in living organisms. Polysaccharides serve for the storage of energy (e.g., starch and glycogen) and as structural components (e.g., cellulose in plants and chitin in arthropods). The 5-carbon monosaccharide ribose is an important component of coenzymes (e.g., ATP, FAD and NAD) and the backbone of the genetic molecule known as RNA. The related deoxyribose is a component of DNA. Saccharides and their derivatives include many other important biomolecules that play key roles in the immune system, fertilization, preventing pathogenesis, blood clotting, and development.In food science and in many informal contexts, the term carbohydrate often means any food that is particularly rich in the complex carbohydrate starch (such as cereals, bread and pasta) or simple carbohydrates, such as sugar (found in candy, jams, and desserts).