SBI 4U biochem 1
... • contain the elements carbon, hydrogen, oxygen, and nitrogen • composed of MANY amino acid subunits • It is the sequence of the amino acid that forms the primary structure of proteins. • The basic amino acid form has a carboxyl group on one end, a methyl group that only has one hydrogen in the midd ...
... • contain the elements carbon, hydrogen, oxygen, and nitrogen • composed of MANY amino acid subunits • It is the sequence of the amino acid that forms the primary structure of proteins. • The basic amino acid form has a carboxyl group on one end, a methyl group that only has one hydrogen in the midd ...
3.1 Life`s molecular diversity is based on the properties of carbon
... small group of monomers. For example, – proteins are made from only 20 different amino acids and – DNA is built from just four kinds of nucleotides. ...
... small group of monomers. For example, – proteins are made from only 20 different amino acids and – DNA is built from just four kinds of nucleotides. ...
Chap 3 Review Questions
... Why do cows have the ability to breakdown cellulose into glucose and humans can not digest cellulose? What are the two main functions of carbohydrates in a living system? Give an example of each. Which type of lipid is most important in biological membranes? a. fats b. steroids c. phospholipids d. o ...
... Why do cows have the ability to breakdown cellulose into glucose and humans can not digest cellulose? What are the two main functions of carbohydrates in a living system? Give an example of each. Which type of lipid is most important in biological membranes? a. fats b. steroids c. phospholipids d. o ...
Chapter 5: Structure and Function of Large Biological Molecules
... 14. Since the monomers in the reaction from #7 are monosaccharides, the polymer is a disaccharide. Name three disaccharides with the formula C12H22O11. Disaccharide ...
... 14. Since the monomers in the reaction from #7 are monosaccharides, the polymer is a disaccharide. Name three disaccharides with the formula C12H22O11. Disaccharide ...
CHAPTER 5
... simple sugars PROPERTIES: • Hydrocarbon chains with hydroxyl groups • Polar molecules • General formula = (CH2O)n (n=3-7) • Role = fuel for cellular work (cellular respiration) • Serves as the carbon skeleton for other types of monomers (ex. Amino acids) • Component of nucleotides (ribose/deoxyribos ...
... simple sugars PROPERTIES: • Hydrocarbon chains with hydroxyl groups • Polar molecules • General formula = (CH2O)n (n=3-7) • Role = fuel for cellular work (cellular respiration) • Serves as the carbon skeleton for other types of monomers (ex. Amino acids) • Component of nucleotides (ribose/deoxyribos ...
Tasty Models2
... 1. Examine the study of gumdrops that you will be using to assemble your molecular models. Now, consider the formula of glucose, C6H12O6. Based on this formula, how should you assign specific colors to the component atoms? (The most common color should be assigned to hydrogen, since hydrogen atoms a ...
... 1. Examine the study of gumdrops that you will be using to assemble your molecular models. Now, consider the formula of glucose, C6H12O6. Based on this formula, how should you assign specific colors to the component atoms? (The most common color should be assigned to hydrogen, since hydrogen atoms a ...
Double bonds
... A ketone and an aldehyde may be structural isomers with different properties, as is the case for acetone and propanal. ...
... A ketone and an aldehyde may be structural isomers with different properties, as is the case for acetone and propanal. ...
Ch - Plain Local Schools
... A. Monomers- small molecular units B. Polymers- large chains of monomers in various shapes C. Life’s major Polymers: carbohydrates, lipids, proteins, and nucleic acids III. Building and Breaking Polymers A. Dehydration Synthesis- to lengthen a polymer chain 1. A hydrogen atom and a hydroxyl group fr ...
... A. Monomers- small molecular units B. Polymers- large chains of monomers in various shapes C. Life’s major Polymers: carbohydrates, lipids, proteins, and nucleic acids III. Building and Breaking Polymers A. Dehydration Synthesis- to lengthen a polymer chain 1. A hydrogen atom and a hydroxyl group fr ...
Week 3 - Carbohydrates - Sugars
... Glucose in bloodstream, cells use Liver converts fructose & galactose to glucose Therefore, all… Polysaccharides, disaccharides, monosaccharides become glucose ...
... Glucose in bloodstream, cells use Liver converts fructose & galactose to glucose Therefore, all… Polysaccharides, disaccharides, monosaccharides become glucose ...
Structure of Organic Compounds
... • Functional groups determine many of the properties of organic compounds. • 3 Types to Know: Amine (NH2), Carboxyl (COOH), Hydroxyl (OH). ...
... • Functional groups determine many of the properties of organic compounds. • 3 Types to Know: Amine (NH2), Carboxyl (COOH), Hydroxyl (OH). ...
NAT 5 Unit 2 Natures Chem Booklet 2 Everyday
... breaking down carbohydrates (using oxygen) to give carbon dioxide and water. state that glucose is the carbohydrate which reacts with oxygen during respiration State that carbohydrates release energy, producing carbon dioxide and water when burned. Explain why the production of carbon dioxide and wa ...
... breaking down carbohydrates (using oxygen) to give carbon dioxide and water. state that glucose is the carbohydrate which reacts with oxygen during respiration State that carbohydrates release energy, producing carbon dioxide and water when burned. Explain why the production of carbon dioxide and wa ...
1. a. False – the carbonyl groups point toward the C
... titration curve could have come from the C-terminal half of the tetrapeptide, which would mean the latter part of the sequence is Arg-Asn. The right titration curve also shows 3 ionizable groups, with pKa’s at 3, 8, and 10.5, corresponding to C-term, N-term, and Tyr or Lys side chain. If this curve ...
... titration curve could have come from the C-terminal half of the tetrapeptide, which would mean the latter part of the sequence is Arg-Asn. The right titration curve also shows 3 ionizable groups, with pKa’s at 3, 8, and 10.5, corresponding to C-term, N-term, and Tyr or Lys side chain. If this curve ...
Ch_2
... • chemical compound: one or two elements joined chemically in a specific ratio • organic compound: compounds found in living things Proteins - made of carbon, oxygen, hydrogen, nitrogen, and some of them also of sulfur - for build up and repair of tissues - found in foods such as meat, eggs, beans, ...
... • chemical compound: one or two elements joined chemically in a specific ratio • organic compound: compounds found in living things Proteins - made of carbon, oxygen, hydrogen, nitrogen, and some of them also of sulfur - for build up and repair of tissues - found in foods such as meat, eggs, beans, ...
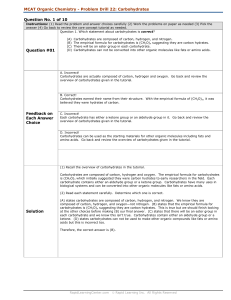
MCAT Organic Chemistry - Problem Drill 22: Carbohydrates
... There are 6 carbons present so it is a hexose. Check the anomeric carbon. If there is a hemiacetal present, then the carbohydrate is an aldose. If there is a acetal present, then the carbohydrate is a ketose. Here, a hemiacetal is present so it is an aldose. The statement is correct. (C) states the ...
... There are 6 carbons present so it is a hexose. Check the anomeric carbon. If there is a hemiacetal present, then the carbohydrate is an aldose. If there is a acetal present, then the carbohydrate is a ketose. Here, a hemiacetal is present so it is an aldose. The statement is correct. (C) states the ...
Organic/Biological Chemistry
... Write symbols for the atoms and guess skeleton structure [ define a central atom ]. Place a pair of electrons in each bond. Complete octets of surrounding atoms. [ H = 2 only ] Place leftover electrons in pairs on the central atom. If there are not enough electrons to give the central atom an octet, ...
... Write symbols for the atoms and guess skeleton structure [ define a central atom ]. Place a pair of electrons in each bond. Complete octets of surrounding atoms. [ H = 2 only ] Place leftover electrons in pairs on the central atom. If there are not enough electrons to give the central atom an octet, ...
essential fatty acid
... DISACCHARIDES Sucrose, maltose and lactose. Sucrose – glucose + fructose, found in sugar cane and sugar beet. Maltose – glucose + glucose, malt sugar – hydrolysis of starch. Lactose – glucose + galactose, milk sugar. POLYSACCHARIDES Starch, cellulose, glycogen and dextrin. ...
... DISACCHARIDES Sucrose, maltose and lactose. Sucrose – glucose + fructose, found in sugar cane and sugar beet. Maltose – glucose + glucose, malt sugar – hydrolysis of starch. Lactose – glucose + galactose, milk sugar. POLYSACCHARIDES Starch, cellulose, glycogen and dextrin. ...
Basic Structure of Amino acid
... The four main classes of organic compounds (carbohydrates, lipids, proteins, and nucleic acids) that are essential to the proper functioning of all living things are known as polymers or macromolecules. All of these compounds are built primarily of carbon, hydrogen, and oxygen but in different ratio ...
... The four main classes of organic compounds (carbohydrates, lipids, proteins, and nucleic acids) that are essential to the proper functioning of all living things are known as polymers or macromolecules. All of these compounds are built primarily of carbon, hydrogen, and oxygen but in different ratio ...
Macromolecules ppt
... carbon, hydrogen, oxygen and nitrogen. Other elements are found in proteins in very small amounts. Protein molecules are constructed from building blocks called amino acids. There are twenty different kinds of amino acids. As amino acids are joined to each other with special covalent peptide bonds, ...
... carbon, hydrogen, oxygen and nitrogen. Other elements are found in proteins in very small amounts. Protein molecules are constructed from building blocks called amino acids. There are twenty different kinds of amino acids. As amino acids are joined to each other with special covalent peptide bonds, ...
Macromolecules 1. If you remove all of the functional groups from an
... 28. Molecules that have the same chemical formula but have different molecular structures are called A) isotopes B) ions C) structural isotopes D) isomers E) both a and c 29. A fatty acid is said to be saturated if: A) one end of the molecule is hydrophilic while the other end is hydrophobic. B) it ...
... 28. Molecules that have the same chemical formula but have different molecular structures are called A) isotopes B) ions C) structural isotopes D) isomers E) both a and c 29. A fatty acid is said to be saturated if: A) one end of the molecule is hydrophilic while the other end is hydrophobic. B) it ...
Chapter 3 - HCC Learning Web
... Why do cows have the ability to breakdown cellulose into glucose and humans can not digest cellulose? What are the two main functions of carbohydrates in a living system? Give an example of each. Which type of lipid is most important in biological membranes? a. fats b. steroids c. phospholipids d. o ...
... Why do cows have the ability to breakdown cellulose into glucose and humans can not digest cellulose? What are the two main functions of carbohydrates in a living system? Give an example of each. Which type of lipid is most important in biological membranes? a. fats b. steroids c. phospholipids d. o ...
Carbohydrates
... Fructose is the fruit sugar, fructose tastes the sweetest of all the sugars—it is what commonly sweetens colas and other soft drinks. It occurs naturally in fruits and vegetables. Food manufacturers use high-fructose corn syrup as an additive to sweeten many foods, including soft drinks, desserts, c ...
... Fructose is the fruit sugar, fructose tastes the sweetest of all the sugars—it is what commonly sweetens colas and other soft drinks. It occurs naturally in fruits and vegetables. Food manufacturers use high-fructose corn syrup as an additive to sweeten many foods, including soft drinks, desserts, c ...
Body Systems Review Sheet 2013
... terms of oxygen and carbon dioxide and glucose levels) to the following activities: exercise, holding your breath, hyperventilating, and breathing into a paper bag. 4. Explain why a physically fit person typically has a lower resting heart rate and their heart rate quickly returns to rest after exer ...
... terms of oxygen and carbon dioxide and glucose levels) to the following activities: exercise, holding your breath, hyperventilating, and breathing into a paper bag. 4. Explain why a physically fit person typically has a lower resting heart rate and their heart rate quickly returns to rest after exer ...
Carbohydrates
... – Supply materials for the synthesis of other biochemical substances – Form part of the structural framework for DNA and RNA molecules – When linked to lipids, they form structural components of cell membranes – When linked to proteins, they participate in cell-cell and cell-molecule recognition pro ...
... – Supply materials for the synthesis of other biochemical substances – Form part of the structural framework for DNA and RNA molecules – When linked to lipids, they form structural components of cell membranes – When linked to proteins, they participate in cell-cell and cell-molecule recognition pro ...
Carbohydrate
A carbohydrate is a biological molecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen:oxygen atom ratio of 2:1 (as in water); in other words, with the empirical formula Cm(H2O)n (where m could be different from n). Some exceptions exist; for example, deoxyribose, a sugar component of DNA, has the empirical formula C5H10O4. Carbohydrates are technically hydrates of carbon; structurally it is more accurate to view them as polyhydroxy aldehydes and ketones.The term is most common in biochemistry, where it is a synonym of saccharide, a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. In general, the monosaccharides and disaccharides, which are smaller (lower molecular weight) carbohydrates, are commonly referred to as sugars. The word saccharide comes from the Greek word σάκχαρον (sákkharon), meaning ""sugar."" While the scientific nomenclature of carbohydrates is complex, the names of the monosaccharides and disaccharides very often end in the suffix -ose. For example, grape sugar is the monosaccharide glucose, cane sugar is the disaccharide sucrose and milk sugar is the disaccharide lactose (see illustration).Carbohydrates perform numerous roles in living organisms. Polysaccharides serve for the storage of energy (e.g., starch and glycogen) and as structural components (e.g., cellulose in plants and chitin in arthropods). The 5-carbon monosaccharide ribose is an important component of coenzymes (e.g., ATP, FAD and NAD) and the backbone of the genetic molecule known as RNA. The related deoxyribose is a component of DNA. Saccharides and their derivatives include many other important biomolecules that play key roles in the immune system, fertilization, preventing pathogenesis, blood clotting, and development.In food science and in many informal contexts, the term carbohydrate often means any food that is particularly rich in the complex carbohydrate starch (such as cereals, bread and pasta) or simple carbohydrates, such as sugar (found in candy, jams, and desserts).