Writing Chemical Equations
... equation in which the formulas for reacting substances (reactants) are written to the left. An arrow separates these from the formulas for products. • 2Al(s) + 3Br2(l) → 2 AlBr3(s) ...
... equation in which the formulas for reacting substances (reactants) are written to the left. An arrow separates these from the formulas for products. • 2Al(s) + 3Br2(l) → 2 AlBr3(s) ...
Chapter 1 Introduction: Matter and Measurement
... random motion unless constrained. Use Ar atom as an example. Draw diagrams of s, l, and g Ar and use them to show how this theory explains all of the observations in chart above describing properties of states of matter ...
... random motion unless constrained. Use Ar atom as an example. Draw diagrams of s, l, and g Ar and use them to show how this theory explains all of the observations in chart above describing properties of states of matter ...
Spring Benchmark Exam
... temperature holds 525 grams of helium. What type of electronic probe could be used to determine the pressure inside the balloon? A B C D ...
... temperature holds 525 grams of helium. What type of electronic probe could be used to determine the pressure inside the balloon? A B C D ...
Name: Block:______ Date:______ ChemThink: Particulate
... Complete the Question Set for the Particulate Nature of Matter. This is graded. Finish questions successfully, you will need to answer 10 questions correctly before missing 3 questions! Follow the directions. Read carefully, Note: If you have to redo the question set more than 3 times, you need to: ...
... Complete the Question Set for the Particulate Nature of Matter. This is graded. Finish questions successfully, you will need to answer 10 questions correctly before missing 3 questions! Follow the directions. Read carefully, Note: If you have to redo the question set more than 3 times, you need to: ...
Chapter 12 - Midway ISD
... Sect. 12-1: Liquids Properties of liquids Definite volume Takes shape of its container Properties can be explained by the kinetic molecular theory (KMT) just like gases were explained Fluid – has the ability to flow ...
... Sect. 12-1: Liquids Properties of liquids Definite volume Takes shape of its container Properties can be explained by the kinetic molecular theory (KMT) just like gases were explained Fluid – has the ability to flow ...
Let’s talk Chemistry!
... In what form of matter do the molecules have the greatest attractions to one another? Solids Often atoms join so that each atom will have A full outer energy level (gaining or losing electrons) When two hydrogen atoms bond, the positive nucleus of one atom attracts the Negative electron of the othe ...
... In what form of matter do the molecules have the greatest attractions to one another? Solids Often atoms join so that each atom will have A full outer energy level (gaining or losing electrons) When two hydrogen atoms bond, the positive nucleus of one atom attracts the Negative electron of the othe ...
Notes matter energy
... number and type of atoms in a molecule. For example, H2SO4 (sulfuric acid) is the formula for a molecule because it consists of only nonmetals. The molecule is made up of 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms (and 7 total atoms). Subscripts indicate the number of atoms in the formula ( ...
... number and type of atoms in a molecule. For example, H2SO4 (sulfuric acid) is the formula for a molecule because it consists of only nonmetals. The molecule is made up of 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms (and 7 total atoms). Subscripts indicate the number of atoms in the formula ( ...
Intro to matter clas.. - hrsbstaff.ednet.ns.ca
... A mixture is matter that does not have definite composition, one mixture of salt and water does not have the same composition as another mixture of salt and water. The two solutions have differing amounts of salt .Example: solution one has 5 grams salt in a litre of water and solution two contains 1 ...
... A mixture is matter that does not have definite composition, one mixture of salt and water does not have the same composition as another mixture of salt and water. The two solutions have differing amounts of salt .Example: solution one has 5 grams salt in a litre of water and solution two contains 1 ...
September 26, 2012
... a. More than one phase (like oil and water) State: We need at least two intensive properties to define the state of a pure substance. Temperature: ...
... a. More than one phase (like oil and water) State: We need at least two intensive properties to define the state of a pure substance. Temperature: ...
Stuff Matters Handout
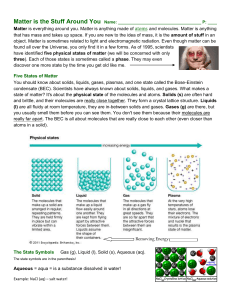
... three). Each of those states is sometimes called a phase. They may even discover one more state by the time you get old like me. Five States of Matter You should know about solids, liquids, gases, plasmas, and one state called the Bose-Einstein condensate (BEC). Scientists have always known about so ...
... three). Each of those states is sometimes called a phase. They may even discover one more state by the time you get old like me. Five States of Matter You should know about solids, liquids, gases, plasmas, and one state called the Bose-Einstein condensate (BEC). Scientists have always known about so ...
NAME
... CHEMISTRY I CHAPTER 3 PROBLEM SET #1 (Questions 1-5) Define the following terms in your own words: ...
... CHEMISTRY I CHAPTER 3 PROBLEM SET #1 (Questions 1-5) Define the following terms in your own words: ...
Physics Close to Absolute Zero Temperature
... Dr Shizhong Zhang has been working in this general area for the past several years, making use of a new type of system, consisting of ultracold atomic gases confined in a magnetic or an optical trap. Unlike electrons in solid state materials, these much larger neutral entities move with a velocity t ...
... Dr Shizhong Zhang has been working in this general area for the past several years, making use of a new type of system, consisting of ultracold atomic gases confined in a magnetic or an optical trap. Unlike electrons in solid state materials, these much larger neutral entities move with a velocity t ...
Bulk Properties and Neutron Diffraction of the Magnetic
... Physik Department E21, TU München, D-85748 Garching, Germany ...
... Physik Department E21, TU München, D-85748 Garching, Germany ...
Basic Chemistry Notes II
... 3. The atomic number is the number of protons B. Neutrons 1. Found in nucleus 2. No charge 3. Can be found by subtracting the atomic number from the atomic weight C. Electrons 1. Found outside of nucleus in “shells” 2. Have a negative charge 3. Valence electrons – outermost electron shell. Most impo ...
... 3. The atomic number is the number of protons B. Neutrons 1. Found in nucleus 2. No charge 3. Can be found by subtracting the atomic number from the atomic weight C. Electrons 1. Found outside of nucleus in “shells” 2. Have a negative charge 3. Valence electrons – outermost electron shell. Most impo ...
Chapter 1 Matter and Change
... - substance composed of 2 or more elements that are chemically combined - have unique chemical and physical properties - can be broken down chemically (not physically) - properties of elements that compounds are broken down into do not resemble properties of original ...
... - substance composed of 2 or more elements that are chemically combined - have unique chemical and physical properties - can be broken down chemically (not physically) - properties of elements that compounds are broken down into do not resemble properties of original ...
Honors Chemistry Review Packet KEY
... the same set of intensive properties because they have different chemical compositions. 12. As either heterogeneous or homogeneous 13. Differences in physical properties 15. a) homogeneous, b) heterogeneous, c) homogeneous, d) heterogeneous 21. The liquid was not an element because a solid was lefto ...
... the same set of intensive properties because they have different chemical compositions. 12. As either heterogeneous or homogeneous 13. Differences in physical properties 15. a) homogeneous, b) heterogeneous, c) homogeneous, d) heterogeneous 21. The liquid was not an element because a solid was lefto ...
Inductively-Coupled Plasma (ICP) Excitation Source
... Spark and arc atomic emission spectroscopy Spark or arc atomic emission spectroscopy is used for the analysis of metallic elements in solid samples. For non-conductive materials, the sample is ground with graphite powder to make it conductive. In traditional arc spectroscopy methods, a sample of the ...
... Spark and arc atomic emission spectroscopy Spark or arc atomic emission spectroscopy is used for the analysis of metallic elements in solid samples. For non-conductive materials, the sample is ground with graphite powder to make it conductive. In traditional arc spectroscopy methods, a sample of the ...
Ch. 1-- Matter and Change
... produced are written on the _______ right and are called the “products.” Reactants Products ...
... produced are written on the _______ right and are called the “products.” Reactants Products ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).