Chapter 6 – How Cells Harvest Chemical Energy Standard 1.g
... released in ATP, the rest is released as heat ATP is produced in 2 ways 1. The movement of electrons along an electron transport chain creates a proton gradient across the inner membrane. The protons diffuse back across the membrane through ATP synthase releasing energy that is used to make ATP by 2 ...
... released in ATP, the rest is released as heat ATP is produced in 2 ways 1. The movement of electrons along an electron transport chain creates a proton gradient across the inner membrane. The protons diffuse back across the membrane through ATP synthase releasing energy that is used to make ATP by 2 ...
Lehninger Principles of Biochemistry 5/e
... drop reaction rate is decreased. AMP concentration is more sensitive indicator of cell’s energetic state than is [ATP] AMP-activated protein kinase - regulated by [AMP] - A reduced nutrient supply or by increase exercise cause the rise in [AMP] - increase glucose uptake, activates glycolysis and f ...
... drop reaction rate is decreased. AMP concentration is more sensitive indicator of cell’s energetic state than is [ATP] AMP-activated protein kinase - regulated by [AMP] - A reduced nutrient supply or by increase exercise cause the rise in [AMP] - increase glucose uptake, activates glycolysis and f ...
Macronutrients
... Adenosine triphosphate Can be easily transformed to ADP (releasing energy) and back to ATP, making it an effective molecule for this process ...
... Adenosine triphosphate Can be easily transformed to ADP (releasing energy) and back to ATP, making it an effective molecule for this process ...
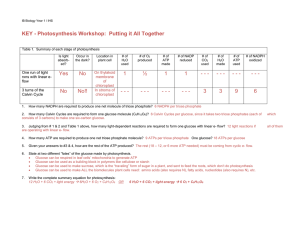
KEY - Photosynthesis Workshop: Putting it All Together
... consists of 3 carbons) to make one six-carbon glucose. ...
... consists of 3 carbons) to make one six-carbon glucose. ...
Regulation of carbohydrate metabolism
... 1. 3 key enzymes for the regulation of glycolysis (their activation). The role of Fructose 2,6-P in the regulation of glycolysis and gluconeogenesis. 2. 3 key sites for the regulation of gluconeogenesis (their activation). 3. The signal pathway for the activation of glycogen degradation by glucagon. ...
... 1. 3 key enzymes for the regulation of glycolysis (their activation). The role of Fructose 2,6-P in the regulation of glycolysis and gluconeogenesis. 2. 3 key sites for the regulation of gluconeogenesis (their activation). 3. The signal pathway for the activation of glycogen degradation by glucagon. ...
Exam Review 2 10/2/16
... B. Light is captured in the head region of the chlorophyll C. Chlorophyll absorbs light at all wavelengths of the visible spectrum D. The tail region of chlorophyll has no known function 43. Where does the Calvin cycle take place? A. Thylakoid membrane B. Cytoplasm C. Stroma D. Granum 44. The replic ...
... B. Light is captured in the head region of the chlorophyll C. Chlorophyll absorbs light at all wavelengths of the visible spectrum D. The tail region of chlorophyll has no known function 43. Where does the Calvin cycle take place? A. Thylakoid membrane B. Cytoplasm C. Stroma D. Granum 44. The replic ...
Respiration Respiration Respiration
... ΔG = -686kcal/mol of glucose ΔG can be even higher than this in a cell This large amount of energy must be released in small steps rather than all at once. ...
... ΔG = -686kcal/mol of glucose ΔG can be even higher than this in a cell This large amount of energy must be released in small steps rather than all at once. ...
How Cells Harvest Energy
... transferring a phosphate directly to ADP from another molecule 2. oxidative phosphorylation – use of ATP synthase and energy derived from a proton (H+) gradient to make ATP ...
... transferring a phosphate directly to ADP from another molecule 2. oxidative phosphorylation – use of ATP synthase and energy derived from a proton (H+) gradient to make ATP ...
BCHM 562, Biochemistry II
... to the FADH2, whereby it accepts two H atoms. 3. FMN functions as prosthetic group of various oxidoreductases such as NADH dehydrogenase. 4. During catalytic cycle, the reversible interconversion of oxidized (FMN), semiquinone (FMNH•) and reduced (FMNH2) forms occurs. 5. FMN is a stronger oxidizing ...
... to the FADH2, whereby it accepts two H atoms. 3. FMN functions as prosthetic group of various oxidoreductases such as NADH dehydrogenase. 4. During catalytic cycle, the reversible interconversion of oxidized (FMN), semiquinone (FMNH•) and reduced (FMNH2) forms occurs. 5. FMN is a stronger oxidizing ...
Cellular Respiration
... Producers change solar energy to chemical energy of organic molecules – glucose, amino acids Animals and also plants break chemical bonds of sugar molecules and make ATP. Use ATP for all cellular functions 4 Main Step of Cellular Respiration Glycolysis: Glucose + 2NAD + 2ADP 2 Pyruvate + 2NADH + 2 ...
... Producers change solar energy to chemical energy of organic molecules – glucose, amino acids Animals and also plants break chemical bonds of sugar molecules and make ATP. Use ATP for all cellular functions 4 Main Step of Cellular Respiration Glycolysis: Glucose + 2NAD + 2ADP 2 Pyruvate + 2NADH + 2 ...
BY 330 Summer 2015Mock Exam 2 Ten molecules of
... pyruvate through glycolysis. What is the net ATP production? How many molecules of NADH are produced? How many molecules of CO2 are produced? How many molecules of pyruvate are formed? Show the pathway for this conversion, including all intermediates and energy production sites. (I won’t show the pa ...
... pyruvate through glycolysis. What is the net ATP production? How many molecules of NADH are produced? How many molecules of CO2 are produced? How many molecules of pyruvate are formed? Show the pathway for this conversion, including all intermediates and energy production sites. (I won’t show the pa ...
9.3 student notes
... • Proteins and nucleic acids can also be used to make ATP, but they are usually used for building important cell parts. ...
... • Proteins and nucleic acids can also be used to make ATP, but they are usually used for building important cell parts. ...
Kevin Ahern's Biochemistry (BB 450/550) at Oregon State University
... reduction and requires ATP and ATP.. There are 10 reactions in glycolysis. Students should know structures of fructose and glucose compounds, glyceraldehyde-3-phosphate, 1,3 BPG, all enzyme names, all molecule names, and reactions I described where the Delta G zero prime is strongly positive, or str ...
... reduction and requires ATP and ATP.. There are 10 reactions in glycolysis. Students should know structures of fructose and glucose compounds, glyceraldehyde-3-phosphate, 1,3 BPG, all enzyme names, all molecule names, and reactions I described where the Delta G zero prime is strongly positive, or str ...
ATP Production
... Some organisms live in an oxygen-free environment. The Kreb’s Cycle and Electron Transport How do they get their energy? ...
... Some organisms live in an oxygen-free environment. The Kreb’s Cycle and Electron Transport How do they get their energy? ...
Lecture 3section7
... Remember pathways are integrated Rates of glycolysis and TCA cycle are matched so that only as much glucose is metabolized to pyruvate as is need to provide Acetyl CoA for the cycles Rate of glycolysis is matched to the TCA cycle by ATP and NADH levels. Also remember the citrate is a negative allost ...
... Remember pathways are integrated Rates of glycolysis and TCA cycle are matched so that only as much glucose is metabolized to pyruvate as is need to provide Acetyl CoA for the cycles Rate of glycolysis is matched to the TCA cycle by ATP and NADH levels. Also remember the citrate is a negative allost ...
Summary of Metabolism
... from chyromicrons and VLDLs as free fatty acids • Once in the cell they are esterified to glycerol backbone. • Glucagon/epinephrine stimulate reverse process ...
... from chyromicrons and VLDLs as free fatty acids • Once in the cell they are esterified to glycerol backbone. • Glucagon/epinephrine stimulate reverse process ...
Metabolism
... The bottom line for beta-oxidation of stearic acid (C18:0) 20 ATP from 8 NADH 12 ATP from 12 FADH2 9 acetyl coA’s through the citric acid cycle: 9 GTP, 67.5 ATP from 27 NADH and 13.5 ATP from 9 FADH2 Minus 2 ATP to start beta oxidation: 120 ATP Fat burns in a flame of carbohydrate Carbohydrate is ne ...
... The bottom line for beta-oxidation of stearic acid (C18:0) 20 ATP from 8 NADH 12 ATP from 12 FADH2 9 acetyl coA’s through the citric acid cycle: 9 GTP, 67.5 ATP from 27 NADH and 13.5 ATP from 9 FADH2 Minus 2 ATP to start beta oxidation: 120 ATP Fat burns in a flame of carbohydrate Carbohydrate is ne ...
Why Fermentation
... Glycolysis and fermentation together only produces 2 ATP. This is not efficient!! ...
... Glycolysis and fermentation together only produces 2 ATP. This is not efficient!! ...
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL
... 1. Fermentation enables some cells to produce ATP without the help of oxygen • Oxidation refers to the loss of electrons to any electron acceptor, not just to oxygen. • In glycolysis, glucose is oxidized to two pyruvate molecules with NAD+ as the oxidizing agent, not O2. • Some energy from this oxi ...
... 1. Fermentation enables some cells to produce ATP without the help of oxygen • Oxidation refers to the loss of electrons to any electron acceptor, not just to oxygen. • In glycolysis, glucose is oxidized to two pyruvate molecules with NAD+ as the oxidizing agent, not O2. • Some energy from this oxi ...
Glycolysis
... Glyceraldehyde-3-phosphate is oxidized to 1,3biphosphoglycerate (1,3-BPG), catalyzed by a dehydrogenase enzyme. Electrons lost during this oxidation are transferred to NAD+, forming NADH, preserving the reducing power (reductive potential) of the electrons for other metabolic reactions. In 1,3-BPG t ...
... Glyceraldehyde-3-phosphate is oxidized to 1,3biphosphoglycerate (1,3-BPG), catalyzed by a dehydrogenase enzyme. Electrons lost during this oxidation are transferred to NAD+, forming NADH, preserving the reducing power (reductive potential) of the electrons for other metabolic reactions. In 1,3-BPG t ...
Related Metabolic Processes
... 1. Fermentation enables some cells to produce ATP without the help of oxygen • Oxidation refers to the loss of electrons to any electron acceptor, not just to oxygen. • In glycolysis, glucose is oxidized to two pyruvate molecules with NAD+ as the oxidizing agent, not O2. • Some energy from this oxi ...
... 1. Fermentation enables some cells to produce ATP without the help of oxygen • Oxidation refers to the loss of electrons to any electron acceptor, not just to oxygen. • In glycolysis, glucose is oxidized to two pyruvate molecules with NAD+ as the oxidizing agent, not O2. • Some energy from this oxi ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑