Ch 9 (primary ppt) - Phillips Scientific Methods
... The role of glycolysis in oxidizing glucose to two molecules of pyruvate. The process that brings pyruvate from the cytosol into the mitochondria and introduces it into the ...
... The role of glycolysis in oxidizing glucose to two molecules of pyruvate. The process that brings pyruvate from the cytosol into the mitochondria and introduces it into the ...
Document
... • This reaction is catalyzed by NO synthase, which is found in many tissues and cell types. ...
... • This reaction is catalyzed by NO synthase, which is found in many tissues and cell types. ...
Metabolism
... levels of glucose-6-phosphate, which prevents the phosphorylation of glucose. • Reaction 3 Phosphofructokinase, an allosteric enzyme, is inhibited by high levels of ATP and activated by high levels of ADP and AMP. • Reaction 10 Pyruvate kinase, another allosteric enzyme is inhibited by high levels o ...
... levels of glucose-6-phosphate, which prevents the phosphorylation of glucose. • Reaction 3 Phosphofructokinase, an allosteric enzyme, is inhibited by high levels of ATP and activated by high levels of ADP and AMP. • Reaction 10 Pyruvate kinase, another allosteric enzyme is inhibited by high levels o ...
4-Carbohydrate metabolism
... Carbohydrates are a superior short-term energy reserve for organisms, because they are much simpler to metabolize than fats or proteins. Carbohydrates are typically stored as long polymers of glucose molecules with Glycosidic bonds for structural support (e.g. chitin, cellulose) or energy storag ...
... Carbohydrates are a superior short-term energy reserve for organisms, because they are much simpler to metabolize than fats or proteins. Carbohydrates are typically stored as long polymers of glucose molecules with Glycosidic bonds for structural support (e.g. chitin, cellulose) or energy storag ...
Glycolysis Reactions
... production of a relatively small amount of ATP. Glycolysis can be carried out anerobically (in the absence of oxygen) and is thus an especially important pathway for organisms that can ferment sugars. For example, glycolysis is the pathway utilized by yeast to produce the alcohol found in beer. Glyc ...
... production of a relatively small amount of ATP. Glycolysis can be carried out anerobically (in the absence of oxygen) and is thus an especially important pathway for organisms that can ferment sugars. For example, glycolysis is the pathway utilized by yeast to produce the alcohol found in beer. Glyc ...
Metabolism
... electron transport system to yield ATP 2 turns of cycle are completed for each glucose molecule 2 ATP + 6 NADH +2 FADH2 ...
... electron transport system to yield ATP 2 turns of cycle are completed for each glucose molecule 2 ATP + 6 NADH +2 FADH2 ...
Time: 1.5 hour
... 23. Anaerobic process after glycolysis is called: (a) TCA (b) Calvin cycle (c) Krebs’ cycle (d) Fermentation 24. The formation of acetyl coenzyme from pyruvic acid is the result of its: (a) Reduction (b) Dehydration (c) Dephosphorylation (d) Oxidative decarboxylation 25. Glycolysis give rise to (a) ...
... 23. Anaerobic process after glycolysis is called: (a) TCA (b) Calvin cycle (c) Krebs’ cycle (d) Fermentation 24. The formation of acetyl coenzyme from pyruvic acid is the result of its: (a) Reduction (b) Dehydration (c) Dephosphorylation (d) Oxidative decarboxylation 25. Glycolysis give rise to (a) ...
2nd Phase of Glycolysis
... histidine to the C2 position of 3-phosphoglycerate to form 2,3-bisphosphoglycerate. 2,3-bisphosphoglycerate then transfers the C3 phosphoryl group to the active site histidine residue to regenerate the active phosphoenzyme and produce 2-phosphoglycerate. Once in every 100 turnovers, the intermediate ...
... histidine to the C2 position of 3-phosphoglycerate to form 2,3-bisphosphoglycerate. 2,3-bisphosphoglycerate then transfers the C3 phosphoryl group to the active site histidine residue to regenerate the active phosphoenzyme and produce 2-phosphoglycerate. Once in every 100 turnovers, the intermediate ...
Carbohydrate metabolism
... a) to pyruvate (Pyr) CH3-CO- COO- under aerobic conditions = aerobic glycolysis b) to lactate CH3-CHOH - COO- when O2 is depleted = anaerobic glycolysis The individual reactions of glycolysis (see Fig. 1): 1. Phosphorylation of Glc to Glc-6-P is the first reaction of glycolysis and it is a regulator ...
... a) to pyruvate (Pyr) CH3-CO- COO- under aerobic conditions = aerobic glycolysis b) to lactate CH3-CHOH - COO- when O2 is depleted = anaerobic glycolysis The individual reactions of glycolysis (see Fig. 1): 1. Phosphorylation of Glc to Glc-6-P is the first reaction of glycolysis and it is a regulator ...
CH 7 Reading Guide 2014
... 4. The following is a generalized formula for a redox reaction: Xe- + Y X + YeDraw an arrow showing which component (X or Y) is oxidized and which is reduced. ______________ is the reducing agent in this reaction, and __________________ is the oxidizing agent. 5. When compounds lose electrons, they ...
... 4. The following is a generalized formula for a redox reaction: Xe- + Y X + YeDraw an arrow showing which component (X or Y) is oxidized and which is reduced. ______________ is the reducing agent in this reaction, and __________________ is the oxidizing agent. 5. When compounds lose electrons, they ...
B324notesTheme 2
... The major allosteric regulatory factor of the two pathways is Fructose 2,6 bisphosphate. Note in Figure 16.7 that PFK-2 and Fructose 2,6-bisphosphatase are on the same peptide and are affected differently by phosphorylation (see below). Interconversion of PFK-2 and Fructose 2,6-bisphosphatase depend ...
... The major allosteric regulatory factor of the two pathways is Fructose 2,6 bisphosphate. Note in Figure 16.7 that PFK-2 and Fructose 2,6-bisphosphatase are on the same peptide and are affected differently by phosphorylation (see below). Interconversion of PFK-2 and Fructose 2,6-bisphosphatase depend ...
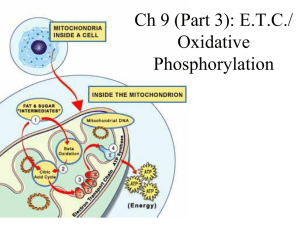
Ch 9: E.T.C./ Oxidative Phosphorylation
... electronegative than the group before it, so the electrons are “pulled downhill” towards OXYGEN (the final electron carrier!) ...
... electronegative than the group before it, so the electrons are “pulled downhill” towards OXYGEN (the final electron carrier!) ...
AP Biology Cellular Respiration Notes 9.1
... production of ATP by chemiosmosis. 1. Electrons are made available in the Citric Acid cycle. 2. The first protein in the ETC is reduced when it accepts e-‘s 3. The proteins of the ETC are arranged by increasing electronegativity 4. The proteins pull the e- back and forth across the membrane “exergon ...
... production of ATP by chemiosmosis. 1. Electrons are made available in the Citric Acid cycle. 2. The first protein in the ETC is reduced when it accepts e-‘s 3. The proteins of the ETC are arranged by increasing electronegativity 4. The proteins pull the e- back and forth across the membrane “exergon ...
Chapter 8
... transferring a phosphate directly to ADP from another molecule 2. oxidative phosphorylation – use of ATP synthase and energy derived from a proton (H+) gradient to make ATP ...
... transferring a phosphate directly to ADP from another molecule 2. oxidative phosphorylation – use of ATP synthase and energy derived from a proton (H+) gradient to make ATP ...
Learning Objectives Chapter 2 Biochem [10-30
... 6. Describe the 2 lipoproteins and how they are cleared Chylomicrons: formed in the intestinal epithelial cells from the products of dietary triacylglycerols; remnants cleared from the body by the liver VLDL: synthesized in the liver; remnants cleared by the liver, or they form low-density lipoprote ...
... 6. Describe the 2 lipoproteins and how they are cleared Chylomicrons: formed in the intestinal epithelial cells from the products of dietary triacylglycerols; remnants cleared from the body by the liver VLDL: synthesized in the liver; remnants cleared by the liver, or they form low-density lipoprote ...
Unfinished business from April 4!
... Static (steady-state) “knowledge units” genome sequence, microarray profile, proteome composition How to understand cellular dynamics? Flux – where to measure, how and what is the most important “link”? Metabolites – intermediates in pathways to end-products (starch, cellulose, proteins, fats, lipid ...
... Static (steady-state) “knowledge units” genome sequence, microarray profile, proteome composition How to understand cellular dynamics? Flux – where to measure, how and what is the most important “link”? Metabolites – intermediates in pathways to end-products (starch, cellulose, proteins, fats, lipid ...
Structural basics of human muscle fructose-1,6
... Structural basics of human muscle fructose-1,6-bisphosphatase activity Jakub Barciszewski Glucose is the main energy source in mammals where its homeostasis in blood is maintained by the balance of catabolic glycolysis on the one site and gluconeogenesis on the other hand. Fructose-1,6-bisphosphatas ...
... Structural basics of human muscle fructose-1,6-bisphosphatase activity Jakub Barciszewski Glucose is the main energy source in mammals where its homeostasis in blood is maintained by the balance of catabolic glycolysis on the one site and gluconeogenesis on the other hand. Fructose-1,6-bisphosphatas ...
fatty acid synthesis
... mammalian cells cannot introduce double bonds more than 9 carbons from carboxyl end. Therefore, linoleate (18:29,12) and -linoleate (18:39,12,15), essential fatty acids, must be obtained from plants. These can however be elongated and additional double bonds added. See fig 16-7 for synthesis of ...
... mammalian cells cannot introduce double bonds more than 9 carbons from carboxyl end. Therefore, linoleate (18:29,12) and -linoleate (18:39,12,15), essential fatty acids, must be obtained from plants. These can however be elongated and additional double bonds added. See fig 16-7 for synthesis of ...
Chapter_9_ppt_FINAL_FINAL_AP_BIO
... force/charge gradient or voltage difference; electropotential. ...
... force/charge gradient or voltage difference; electropotential. ...
Clostridia
... some of the carboxylic acid groups are esterified with methanol. During fermentation, the ester groups are hydrolyzed, and methanol is released ...
... some of the carboxylic acid groups are esterified with methanol. During fermentation, the ester groups are hydrolyzed, and methanol is released ...
Bio 110 S.I. chapters 6 & 7
... pyruvate reduction citric acid cycle electron transport chain fermentation ...
... pyruvate reduction citric acid cycle electron transport chain fermentation ...
Cellular Respiration
... During aerobic respiration, where do the electrons (H+) end up as they are passed from protein to protein? They end up in the loving arms of oxygen. When oxygen accepts electrons, water is made. If oxygen wasn’t there to accept the electrons, the Electron Transport Chain would get backed up, and no ...
... During aerobic respiration, where do the electrons (H+) end up as they are passed from protein to protein? They end up in the loving arms of oxygen. When oxygen accepts electrons, water is made. If oxygen wasn’t there to accept the electrons, the Electron Transport Chain would get backed up, and no ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑