Chapter 6 How Cells Harvest Chemical Energy
... NADH and FADH2 molecules With the help of CoA, the acetyl (two-carbon) compound enters the citric acid cycle – At this point, the acetyl group associates with a fourcarbon molecule forming a six-carbon molecule – The six-carbon molecule then passes through a series of redox reactions that regenera ...
... NADH and FADH2 molecules With the help of CoA, the acetyl (two-carbon) compound enters the citric acid cycle – At this point, the acetyl group associates with a fourcarbon molecule forming a six-carbon molecule – The six-carbon molecule then passes through a series of redox reactions that regenera ...
All the following is correct about ribosomes EXCEPT
... Phospholipids have……………….. attached to glycerol and a phosphate group. a. one fatty acid b. two fatty acids c. three fatty acids d. four fatty acids ...
... Phospholipids have……………….. attached to glycerol and a phosphate group. a. one fatty acid b. two fatty acids c. three fatty acids d. four fatty acids ...
Unit 5
... with biological activity (including proteins, DNA, sugars, and fats). In addition, the energy stored in bonds between the atoms (chemical energy) can be used as sources of energy for life processes. The chemical bonds of food molecules contain energy. Energy is released when the bonds of food molecu ...
... with biological activity (including proteins, DNA, sugars, and fats). In addition, the energy stored in bonds between the atoms (chemical energy) can be used as sources of energy for life processes. The chemical bonds of food molecules contain energy. Energy is released when the bonds of food molecu ...
222 Coenzymes.p65
... 3. The hydrogen atoms are passed to the coenzymes NAD and FAD (flavine adenine dinucleotide), which are thus reduced 4. ATP is produced 5. Oxaloacetate is eventually regenerated, ready to react with more acetate from acetyl coenzyme A ...
... 3. The hydrogen atoms are passed to the coenzymes NAD and FAD (flavine adenine dinucleotide), which are thus reduced 4. ATP is produced 5. Oxaloacetate is eventually regenerated, ready to react with more acetate from acetyl coenzyme A ...
2.1 Carbohydrates - SandyBiology1-2
... • The chemical mechanisms that cells use to make and break polymers are similar for all classes of macromolecules. ...
... • The chemical mechanisms that cells use to make and break polymers are similar for all classes of macromolecules. ...
Organic Chemistry & Carbohydrates: Structure & Function
... asymmetric carbon, resulting in molecules that are mirror images, like left and right hands. The two isomers are designated the L and D isomers from the Latin for left and right (levo and dextro). Enantiomers cannot be ...
... asymmetric carbon, resulting in molecules that are mirror images, like left and right hands. The two isomers are designated the L and D isomers from the Latin for left and right (levo and dextro). Enantiomers cannot be ...
Cell Energy
... ATP: Adenosine Triphosphate • Molecule that delivers immediately available energy to run cellular processes (active transport, movement, mitosis, production of proteins etc.) • All other food/energy molecules (various lipids, carbs, proteins) are converted into ATP through enzyme machinery in cells/ ...
... ATP: Adenosine Triphosphate • Molecule that delivers immediately available energy to run cellular processes (active transport, movement, mitosis, production of proteins etc.) • All other food/energy molecules (various lipids, carbs, proteins) are converted into ATP through enzyme machinery in cells/ ...
Chapter 15 Lecture Notes: Metabolism
... Note that H+ is produced in this reaction. You will see H+ ions as products in many of the reactions in this chapter. Keep in mind that the H+ ions that are produced in aqueous solutions do not remain solvated as isolated ions; they quickly react with water to form H3O+. Alternatively, H+ can react ...
... Note that H+ is produced in this reaction. You will see H+ ions as products in many of the reactions in this chapter. Keep in mind that the H+ ions that are produced in aqueous solutions do not remain solvated as isolated ions; they quickly react with water to form H3O+. Alternatively, H+ can react ...
AP Biology PDQ`s
... Why are they necessary? 4. Is glucose the only molecule that can be catabolized during cellular respiration? Why do we use glucose as the model? 5. Why do hydrogen atoms accompany electrons as they are transferred in biological systems? 6. Why is it thought that glycolysis is the first catabolic pat ...
... Why are they necessary? 4. Is glucose the only molecule that can be catabolized during cellular respiration? Why do we use glucose as the model? 5. Why do hydrogen atoms accompany electrons as they are transferred in biological systems? 6. Why is it thought that glycolysis is the first catabolic pat ...
The Biochemistry of Red blood cells Metabolism and
... • RBCs contain no mitochondria, so there is no respiratory chain, no citric acid cycle, and no oxidation of fatty acids or ketone bodies. • The RBC is highly dependent upon glucose as its energy source. • Energy in the form of ATP is obtained only from the glycolytic breakdown of glucose with the pr ...
... • RBCs contain no mitochondria, so there is no respiratory chain, no citric acid cycle, and no oxidation of fatty acids or ketone bodies. • The RBC is highly dependent upon glucose as its energy source. • Energy in the form of ATP is obtained only from the glycolytic breakdown of glucose with the pr ...
Learning Objectives
... w 1 mole of glycogen produces 3 mole ATP; 1 mole of glucose produces 2 mole of ATP. The difference is due to the fact that it takes 1 mole of ATP to convert glucose to glucose-6-phosphate, where glycogen is converted to glucose-1-phosphate and then to glucose-6-phosphate without the loss of 1 ATP. ...
... w 1 mole of glycogen produces 3 mole ATP; 1 mole of glucose produces 2 mole of ATP. The difference is due to the fact that it takes 1 mole of ATP to convert glucose to glucose-6-phosphate, where glycogen is converted to glucose-1-phosphate and then to glucose-6-phosphate without the loss of 1 ATP. ...
Metabolism
... w 1 mole of glycogen produces 3 mole ATP; 1 mole of glucose produces 2 mole of ATP. The difference is due to the fact that it takes 1 mole of ATP to convert glucose to glucose-6-phosphate, where glycogen is converted to glucose-1-phosphate and then to glucose-6-phosphate without the loss of 1 ATP. ...
... w 1 mole of glycogen produces 3 mole ATP; 1 mole of glucose produces 2 mole of ATP. The difference is due to the fact that it takes 1 mole of ATP to convert glucose to glucose-6-phosphate, where glycogen is converted to glucose-1-phosphate and then to glucose-6-phosphate without the loss of 1 ATP. ...
III. 4. Test Respiració cel·lular
... 27) The free energy for the oxidation of glucose to CO2 and water is -686 kcal/mole and the free energy for the reduction of NAD+ to NADH is +53 kcal/mole. Why are only two molecules of NADH formed during glycolysis when it appears that as many as a dozen could be formed? A) Most of the free energy ...
... 27) The free energy for the oxidation of glucose to CO2 and water is -686 kcal/mole and the free energy for the reduction of NAD+ to NADH is +53 kcal/mole. Why are only two molecules of NADH formed during glycolysis when it appears that as many as a dozen could be formed? A) Most of the free energy ...
Sunday School Jeopardy - Chapman @ Norquay School
... Endocytosis is an example of _______ transport, meaning it requires energy. When the cell takes in solid particles, it is called __________. When it takes in liquid, it is called _____________. ...
... Endocytosis is an example of _______ transport, meaning it requires energy. When the cell takes in solid particles, it is called __________. When it takes in liquid, it is called _____________. ...
Ch. 9: Cellular Respiration
... In presence of O2, pyruvate enters the mitochondrion Before citric acid cycle can begin, pyruvate must be converted to acetyl CoA, which links the cycle to glycolysis Citric acid cycle also called the Krebs cycle ...
... In presence of O2, pyruvate enters the mitochondrion Before citric acid cycle can begin, pyruvate must be converted to acetyl CoA, which links the cycle to glycolysis Citric acid cycle also called the Krebs cycle ...
Pathways that Harvest Chemical Energy (Cellular Respiration)
... Photosynthesis takes place in two stages Light-dependent reactions Pigments capture energy from sunlight (photons of light) and electrons from pigments gain energy Use of light/electron energy to make ATP and to reduce NADP+ (an electron carrier) to NADPH Light-independent reactions (Calvin cycle) U ...
... Photosynthesis takes place in two stages Light-dependent reactions Pigments capture energy from sunlight (photons of light) and electrons from pigments gain energy Use of light/electron energy to make ATP and to reduce NADP+ (an electron carrier) to NADPH Light-independent reactions (Calvin cycle) U ...
Document
... Cell Respiration - series cytoplasmic & mitochondrial - linked enzymatic pathways - stepwise OXIDATION food molecules- makes ATP physiological view: uptake of O2 & release of CO2 ...
... Cell Respiration - series cytoplasmic & mitochondrial - linked enzymatic pathways - stepwise OXIDATION food molecules- makes ATP physiological view: uptake of O2 & release of CO2 ...
Lecture 11 (Parker) - Department of Chemistry ::: CALTECH
... Proton Gradients across membranes are created by the oxida@on of carbon fuels pumping protons out resul@ng in the influx of protons through an ATP-synthesizing enzyme (red complex) and the synthesis of ATP from ADP ...
... Proton Gradients across membranes are created by the oxida@on of carbon fuels pumping protons out resul@ng in the influx of protons through an ATP-synthesizing enzyme (red complex) and the synthesis of ATP from ADP ...
Exam 2 Review Sheet - Iowa State University
... What type of fermentation occurs in animal cells in the absence of oxygen? Lactic acid (lactate) fermentation Alcoholic fermentation Galactic fermentation None of these; fermentation can only occur in yeast cells. ...
... What type of fermentation occurs in animal cells in the absence of oxygen? Lactic acid (lactate) fermentation Alcoholic fermentation Galactic fermentation None of these; fermentation can only occur in yeast cells. ...
Crystal Structure and Functional Analysis of Glyceraldehyde
... structures: NAD-free, NAD-bound and sulfate-soaked. Similar to the published GAPDH structure, OsGAPDH shows homotetramer form and each subunit could be seperated to three domains: NAD-binding domain, catalytic domain and S-loop domain. NAD+ bind to OsGAPDH by hydrogen bonds directly and intermediate ...
... structures: NAD-free, NAD-bound and sulfate-soaked. Similar to the published GAPDH structure, OsGAPDH shows homotetramer form and each subunit could be seperated to three domains: NAD-binding domain, catalytic domain and S-loop domain. NAD+ bind to OsGAPDH by hydrogen bonds directly and intermediate ...
EXAM III KEY - the Complex Carbohydrate Research Center
... __T___ 2) Vitamins A, E and K are all isoprenoids. __F___ 3) Transport of ions and small molecules through a bacterial membrane pore requires energy from an ATP to ADP conversion. __T___ 4) The principle advantage of a cascade mechanism in signal transduction is that one molecule of a ligand can aff ...
... __T___ 2) Vitamins A, E and K are all isoprenoids. __F___ 3) Transport of ions and small molecules through a bacterial membrane pore requires energy from an ATP to ADP conversion. __T___ 4) The principle advantage of a cascade mechanism in signal transduction is that one molecule of a ligand can aff ...
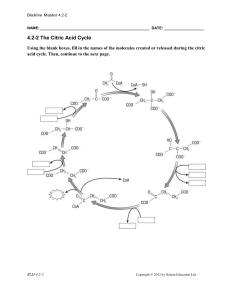
Blackline Master 4.2-2 NAME: DATE: 4.2
... ________________enters the cycle and then combines with ________________ to make the six-carbon compound ________________. During the eight steps of the citric cycle, ________________ undergoes a number of reactions, releasing _______ and ______ in a number of steps. ________________ is eventually c ...
... ________________enters the cycle and then combines with ________________ to make the six-carbon compound ________________. During the eight steps of the citric cycle, ________________ undergoes a number of reactions, releasing _______ and ______ in a number of steps. ________________ is eventually c ...
Flashback - Max-Planck
... process. They discovered 6 of the 15 enzymes that convert molecules in this reaction sequence, and identified about a third of all intermediate products. Two further scientists working in other locations made significant contributions to glycolysis research: Gustav Emden at Goethe University Frankfu ...
... process. They discovered 6 of the 15 enzymes that convert molecules in this reaction sequence, and identified about a third of all intermediate products. Two further scientists working in other locations made significant contributions to glycolysis research: Gustav Emden at Goethe University Frankfu ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑