Thermodynamics (Classical) for Biological Systems Prof. GK
... lactate, coupled with NADH breakdown. This NADH breakdown also happens there and also lactate could be replaced with ethanol in some cases, in some organisms and there also you have an NADH breakdown. This is represented as, pyruvate plus NADH plus H plus giving you lactate plus NAD. What is needed ...
... lactate, coupled with NADH breakdown. This NADH breakdown also happens there and also lactate could be replaced with ethanol in some cases, in some organisms and there also you have an NADH breakdown. This is represented as, pyruvate plus NADH plus H plus giving you lactate plus NAD. What is needed ...
ATP Pool and Growth Yield in Selenomonas
... methods of preparing highly reduced media in bulk and monitoring the cultures. A modification was made to the apparatus so that all residual medium could be removed from the reservoirs on changing from one medium to another of different composition. Ground glass joints in media lines have now been r ...
... methods of preparing highly reduced media in bulk and monitoring the cultures. A modification was made to the apparatus so that all residual medium could be removed from the reservoirs on changing from one medium to another of different composition. Ground glass joints in media lines have now been r ...
AP Biology Summer Assignment Chapter 3 Quiz 2016-17
... the biologist extracted polynucleotides from the cells and separated them into three groups, each containing a range of different polynucleotide lengths. The first group contained the shortest polynucleotides. The second group contained polynucleotides of midrange length, and the third group contain ...
... the biologist extracted polynucleotides from the cells and separated them into three groups, each containing a range of different polynucleotide lengths. The first group contained the shortest polynucleotides. The second group contained polynucleotides of midrange length, and the third group contain ...
digestion of carbohydrates - KSU Faculty Member websites
... The conversion of PEP to pyruvate is catalyzed by pyruvate kinase , the third irriversible reaction of glycolysis . The equilibrium of the pyruvate kinase reaction favors the formation of ATP ( 2 ATP from 2 mols ) . [ This is a second example of substrate-level phosphorylation ] . In liver, Pyruvate ...
... The conversion of PEP to pyruvate is catalyzed by pyruvate kinase , the third irriversible reaction of glycolysis . The equilibrium of the pyruvate kinase reaction favors the formation of ATP ( 2 ATP from 2 mols ) . [ This is a second example of substrate-level phosphorylation ] . In liver, Pyruvate ...
Protein mteabolism
... The rate limiting step in the cycle is the first reaction which is the formation of carbamoyl phosphate from CO2 and NH3 in the presence of carbamoyl phosphate synthetase I (CPSI) which is the rate limiting enzyme in the synthesis. ...
... The rate limiting step in the cycle is the first reaction which is the formation of carbamoyl phosphate from CO2 and NH3 in the presence of carbamoyl phosphate synthetase I (CPSI) which is the rate limiting enzyme in the synthesis. ...
Blood Lactate Concentrations using Fast Glycolysis Comparison of
... two groups that we were collecting from. From the athlete’s group, we concluded that after running the 400 meter and resting their blood lactate would return quicker to their resting state. This is due to their exercise routine training that they partake in. These athletes are using fast glycolysis ...
... two groups that we were collecting from. From the athlete’s group, we concluded that after running the 400 meter and resting their blood lactate would return quicker to their resting state. This is due to their exercise routine training that they partake in. These athletes are using fast glycolysis ...
Metabolism of erythrocytes
... • no ATP production in oxidative phosphorylation • no ability to replace damaged lipids and proteins (low metabolic activities, with no ability to synthesize new proteins or lipids) ...
... • no ATP production in oxidative phosphorylation • no ability to replace damaged lipids and proteins (low metabolic activities, with no ability to synthesize new proteins or lipids) ...
general medicine
... - Principles of the main immunochemical assays used in clinical biochemistry (immunonephelometry and immunoturbidimetry, immunodiffusion methods, immunoelectrophoresis, competitive radioimmunoassays and enzyme immunoassays, ELISA). The oral part of examination ...
... - Principles of the main immunochemical assays used in clinical biochemistry (immunonephelometry and immunoturbidimetry, immunodiffusion methods, immunoelectrophoresis, competitive radioimmunoassays and enzyme immunoassays, ELISA). The oral part of examination ...
otan2hrp
... - Principles of the main immunochemical assays used in clinical biochemistry (immunonephelometry and immunoturbidimetry, immunodiffusion methods, immunoelectrophoresis, competitive radioimmunoassays and enzyme immunoassays, ELISA). The oral part of examination ...
... - Principles of the main immunochemical assays used in clinical biochemistry (immunonephelometry and immunoturbidimetry, immunodiffusion methods, immunoelectrophoresis, competitive radioimmunoassays and enzyme immunoassays, ELISA). The oral part of examination ...
ENZYMES: THE MAJESTIC MOLECULES OF LIFE Part
... There are distinguished, in the active centre, a contact site (anchor site) for binding a substrate, and a catalytic site at which the conversion of the bound substrate takes place. However, this functional differentiation is somewhat arbitrary, since the binding of a substrate at the contact site d ...
... There are distinguished, in the active centre, a contact site (anchor site) for binding a substrate, and a catalytic site at which the conversion of the bound substrate takes place. However, this functional differentiation is somewhat arbitrary, since the binding of a substrate at the contact site d ...
Introduction to Cellular and Molecular Biology (BIOL 190)
... 3. Explain the principles of oxidation and reduction (i.e., redox) and know that electron transfer plays a major role in catabolic reactions and the release of energy 4. Define: reducing and oxidizing agents, electron donor vs. acceptor, electronegativity 5. Recognize NAD+ as an electron carrier, an ...
... 3. Explain the principles of oxidation and reduction (i.e., redox) and know that electron transfer plays a major role in catabolic reactions and the release of energy 4. Define: reducing and oxidizing agents, electron donor vs. acceptor, electronegativity 5. Recognize NAD+ as an electron carrier, an ...
Acyl-CoA
... - Thiolysis (or breaking bonds with –SH group—cf hydrolysis and phosphorolysis) initiated by nucleophilic attack of the thiol group (-SH) of CoA on the keto group within β-ketoacyl-CoA results in the cleavage of Cα-Cβ bond, thereby releasing the first acetyl-CoA (to enter the Krebs cycle) and an out ...
... - Thiolysis (or breaking bonds with –SH group—cf hydrolysis and phosphorolysis) initiated by nucleophilic attack of the thiol group (-SH) of CoA on the keto group within β-ketoacyl-CoA results in the cleavage of Cα-Cβ bond, thereby releasing the first acetyl-CoA (to enter the Krebs cycle) and an out ...
Tutorial Kit (Biochemistry-300 L)
... non-consumable substances that reduce the activation energy necessary for a chemical reaction to occur. Enzymes are highly specific to the reactions they catalyze. They are of vital importance for life because most chemical reactions of the cells and tissues are catalyzed by enzymes. Without enzymat ...
... non-consumable substances that reduce the activation energy necessary for a chemical reaction to occur. Enzymes are highly specific to the reactions they catalyze. They are of vital importance for life because most chemical reactions of the cells and tissues are catalyzed by enzymes. Without enzymat ...
How Cells Obtain Energy Cell Respiration
... Occurs when cells lack O2 or can’t utilize O2 O2 was the final electron acceptor in aerobic cell respiration Lack of O2 or the inability to use O 2 forces some cells to either create their own final electron acceptor (endogenous electron acceptor) or use a compound acquired from outside the cell (ex ...
... Occurs when cells lack O2 or can’t utilize O2 O2 was the final electron acceptor in aerobic cell respiration Lack of O2 or the inability to use O 2 forces some cells to either create their own final electron acceptor (endogenous electron acceptor) or use a compound acquired from outside the cell (ex ...
Quiz - Columbus Labs
... In this diagram of the glycogen phosphorylase dimer, the phosphorylation site (Ser14) and the allosteric (AMP) site face the viewer. Access to the catalytic site is from the opposite side of the protein. The diagram shows the major conformational change that occurs in the N-terminal residues upon ph ...
... In this diagram of the glycogen phosphorylase dimer, the phosphorylation site (Ser14) and the allosteric (AMP) site face the viewer. Access to the catalytic site is from the opposite side of the protein. The diagram shows the major conformational change that occurs in the N-terminal residues upon ph ...
BACK TO GAME
... Abnormal fat accumulation in the liver stems from ________. a. increased uptake of amino acids b. an acetyl CoA driven increase in fatty acid ...
... Abnormal fat accumulation in the liver stems from ________. a. increased uptake of amino acids b. an acetyl CoA driven increase in fatty acid ...
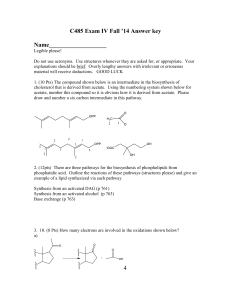
C485 Exam I
... biosynthetic pathway for GMP biosynthesis. You must show all reactions and include all reactants and products. Figure 25.6 and 25.7 7. (12 pts) The carbon backbone of ceramide and sphingosines is assembled in a carboncarbon bond forming reaction. Show the precursors for this reaction, the cofactor r ...
... biosynthetic pathway for GMP biosynthesis. You must show all reactions and include all reactants and products. Figure 25.6 and 25.7 7. (12 pts) The carbon backbone of ceramide and sphingosines is assembled in a carboncarbon bond forming reaction. Show the precursors for this reaction, the cofactor r ...
Fatty Acid Synthesis
... d. Total acetyl-CoA used for priming & for syntheisis of malonate, a + b(c): 8 2- a. How many ~P bonds of ATP used for synthesis of each malonate? 1 b. Total ~P bonds of ATP used for synthesis of one 16-C palmitate, 2a(1c): 7 3- a. How many NADPH used per reaction cycle? 2 b. Total NADPH used per sy ...
... d. Total acetyl-CoA used for priming & for syntheisis of malonate, a + b(c): 8 2- a. How many ~P bonds of ATP used for synthesis of each malonate? 1 b. Total ~P bonds of ATP used for synthesis of one 16-C palmitate, 2a(1c): 7 3- a. How many NADPH used per reaction cycle? 2 b. Total NADPH used per sy ...
Mitochondrium
... Mch. Respiratory chain – moves electrons - pumps H+ into intermembrane space Mch. ATP synthase works also as a H+ pump. ...
... Mch. Respiratory chain – moves electrons - pumps H+ into intermembrane space Mch. ATP synthase works also as a H+ pump. ...
Lactate Inflection Point & Recovery
... inflection point, the more rapid the fatigue This fatigue is generally considered to be a consequence of a greater reliance on the anaerobic systems to supply the adenosine triphosphate (ATP) and the resultant accumulation of the by-products of anaerobic metabolism Lactic acid and hydrogen ions ...
... inflection point, the more rapid the fatigue This fatigue is generally considered to be a consequence of a greater reliance on the anaerobic systems to supply the adenosine triphosphate (ATP) and the resultant accumulation of the by-products of anaerobic metabolism Lactic acid and hydrogen ions ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑