protein pwrpt - Malibu High School
... Nails Outer layers of skin Muscle tissue Bone (the rubbery inner structure) • Bone marrow • Red Blood Cells ...
... Nails Outer layers of skin Muscle tissue Bone (the rubbery inner structure) • Bone marrow • Red Blood Cells ...
lecture5lifes_chemical_basis
... The helical content of a protein may vary anywhere between 0% to 100%. 75% of AAs in Ferritin, an iron storage protein is in alpha-helices. α-helices are usually less than 45Å long. However, two or more α-helices can entwine to form a very stable structure, which can have a length of 1000Å or more. ...
... The helical content of a protein may vary anywhere between 0% to 100%. 75% of AAs in Ferritin, an iron storage protein is in alpha-helices. α-helices are usually less than 45Å long. However, two or more α-helices can entwine to form a very stable structure, which can have a length of 1000Å or more. ...
Slide 1
... • Free amino acids vs. polymerized – Side chains may have different pKas • pKa affected by charges on amino/carboxyl groups • pKa may be affected by interactions with other side chains in the larger molecule ...
... • Free amino acids vs. polymerized – Side chains may have different pKas • pKa affected by charges on amino/carboxyl groups • pKa may be affected by interactions with other side chains in the larger molecule ...
Slide () - Anesthesiology - American Society of Anesthesiologists
... catabolized, releasing amino acids into circulation (including glutamine, alanine, and the branched chain amino acids [BCAAs]), while hepatic amino acid uptake is enhanced. This allows for reprioritization of protein synthesis to acute phase reactants and the production of glucose via gluconeogenesi ...
... catabolized, releasing amino acids into circulation (including glutamine, alanine, and the branched chain amino acids [BCAAs]), while hepatic amino acid uptake is enhanced. This allows for reprioritization of protein synthesis to acute phase reactants and the production of glucose via gluconeogenesi ...
proteinS
... Amino acids are derivatives of carboxylic acids formed by substitution of -hydrogen for amino functional group. ...
... Amino acids are derivatives of carboxylic acids formed by substitution of -hydrogen for amino functional group. ...
Honors Biology Name Biochemistry Exam Review #1 Period _____
... The material an enzyme works on is called the substrates. The pocket or groove where the substrate fits into on the enzyme is called the active site. (See diagram in enzyme notes for enzyme structure) Enzymes are named for the substrate that they work with. Names usually end in –ase (ex. Lactase, He ...
... The material an enzyme works on is called the substrates. The pocket or groove where the substrate fits into on the enzyme is called the active site. (See diagram in enzyme notes for enzyme structure) Enzymes are named for the substrate that they work with. Names usually end in –ase (ex. Lactase, He ...
9AD Biomolecules
... and carry out activities in the cells. Biomolecules are characterized by unique chemical structures and functions. The building blocks of macromolecules are molecular monomers that include saccharides, fatty acids, amino acids, and nucleotides. Other macromolecules are ATP, hormones, and vitamins. 2 ...
... and carry out activities in the cells. Biomolecules are characterized by unique chemical structures and functions. The building blocks of macromolecules are molecular monomers that include saccharides, fatty acids, amino acids, and nucleotides. Other macromolecules are ATP, hormones, and vitamins. 2 ...
Word - LangdonBiology.org
... profile (unique in shape or chemistry). Therefore, each unique combination of amino acids encodes for a different protein with its own unique role and abilities. ...
... profile (unique in shape or chemistry). Therefore, each unique combination of amino acids encodes for a different protein with its own unique role and abilities. ...
1. Overview
... • Coordinates can be extracted and viewed • Comparisons of structures allows identification of structural motifs • Proteins with similar functions and sequences = homologs ...
... • Coordinates can be extracted and viewed • Comparisons of structures allows identification of structural motifs • Proteins with similar functions and sequences = homologs ...
Chemical Principles
... part of bacterial cell wall part of DNA and RNA (deoxyribose and ribose) ...
... part of bacterial cell wall part of DNA and RNA (deoxyribose and ribose) ...
MBP 1022, LECT 2 DAN_Oct22
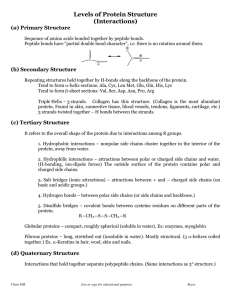
... interactions, disulfide bonds, folding of domains Quarternary; applies to multimeric protein (2 polypep, noncovalent) The sequence of R-groups along the chain is called the primary structure. Secondary structure refers to the local folding of the polypeptide chain. Tertiary structure is the arrangem ...
... interactions, disulfide bonds, folding of domains Quarternary; applies to multimeric protein (2 polypep, noncovalent) The sequence of R-groups along the chain is called the primary structure. Secondary structure refers to the local folding of the polypeptide chain. Tertiary structure is the arrangem ...
Study Guide-Carbon, monomers, polymers, amino acids, proteins
... -What provides appearance and carries out cell activities for an organism? -What provides each protein with a specific function? e. Protein Structure -What happens if you alter shape of protein? -What is denaturation and what causes it? - What are the four levels of organization of a protein? -What ...
... -What provides appearance and carries out cell activities for an organism? -What provides each protein with a specific function? e. Protein Structure -What happens if you alter shape of protein? -What is denaturation and what causes it? - What are the four levels of organization of a protein? -What ...
Chemical Compounds Overview
... d. Dehydration synthesis- Reaction in which water in removed to form a bond, creating a polymer. e. Hydrolysis- Reverse of dehydration synthesis. Polymers are broken down into monomers by adding water. 2. Lipids a. Store energy. Form some membranes. b. Monomer- Glycerol + 3 fatty acids c. Polymer- f ...
... d. Dehydration synthesis- Reaction in which water in removed to form a bond, creating a polymer. e. Hydrolysis- Reverse of dehydration synthesis. Polymers are broken down into monomers by adding water. 2. Lipids a. Store energy. Form some membranes. b. Monomer- Glycerol + 3 fatty acids c. Polymer- f ...
Organic Compounds
... scientists figure out protein folding This game is open to the public The first hundred puzzles are known proteins But many proteins are not decoded and scientists are asking for our help to figure them out http://fold.it/ ...
... scientists figure out protein folding This game is open to the public The first hundred puzzles are known proteins But many proteins are not decoded and scientists are asking for our help to figure them out http://fold.it/ ...
Organic Molecules - University of Dayton
... Q: How do amino acids combine to form proteins? A: Dehydration Synthesis ...
... Q: How do amino acids combine to form proteins? A: Dehydration Synthesis ...
FCS-FS-8. Students will discuss why proteins are important in food
... Help to stabilize pH levels Proteins can supply energy but only when the body is starved of carbohydrates (this is not good for the body) ...
... Help to stabilize pH levels Proteins can supply energy but only when the body is starved of carbohydrates (this is not good for the body) ...
Proteins Review - kehsscience.org
... Act as enzymes to speed up chemical reactions Help transport substances across membranes Store substances the organism needs Help give the cell structure and movement ...
... Act as enzymes to speed up chemical reactions Help transport substances across membranes Store substances the organism needs Help give the cell structure and movement ...
Absolute quantification of proteins and phosphoproteins from cell
... Part 1: AQUA internal standard peptide ...
... Part 1: AQUA internal standard peptide ...
From Genes to Proteins
... • Cells then use tRNA and rRNA to read the instructions on the mRNA molecule and put together the amino acids that make up the protein in a process called translation. ...
... • Cells then use tRNA and rRNA to read the instructions on the mRNA molecule and put together the amino acids that make up the protein in a process called translation. ...
1 - Bulldogbiology.com
... i. Two kinds: DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) Proteins- macromolecules that contain N, C, H, and O a. Polymers of amino acids (monomers) - compounds with an amino group (NH 2 ) on one end; a carboxyl group (COOH) on the other; and an R-Group attached to a central carbon. b. Pr ...
... i. Two kinds: DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) Proteins- macromolecules that contain N, C, H, and O a. Polymers of amino acids (monomers) - compounds with an amino group (NH 2 ) on one end; a carboxyl group (COOH) on the other; and an R-Group attached to a central carbon. b. Pr ...
Document
... G. Importance of lipids- alternate energy source other than monosaccharides; fats are lighter than polysacc. and take up less space ...
... G. Importance of lipids- alternate energy source other than monosaccharides; fats are lighter than polysacc. and take up less space ...
AASK Additional Activities
... a shape that simultaneously satisfies all the 4 principles of chemistry. This is a good teaching moment in that the teacher can use these examples to emphasize that such proteins would not be selected from the enormous pool of possible protein sequences. How can students arrive at a perfectly optimi ...
... a shape that simultaneously satisfies all the 4 principles of chemistry. This is a good teaching moment in that the teacher can use these examples to emphasize that such proteins would not be selected from the enormous pool of possible protein sequences. How can students arrive at a perfectly optimi ...
Lecture 4
... Fibrous proteins are formed from long polypeptide chains that are arranged parallel or nearly parallel to one another. Fibrous polypeptide chains form long strands or sheets and because of many hydrophobic amino acid residues, they are water insoluble but strong and flexible. These long fibers or sh ...
... Fibrous proteins are formed from long polypeptide chains that are arranged parallel or nearly parallel to one another. Fibrous polypeptide chains form long strands or sheets and because of many hydrophobic amino acid residues, they are water insoluble but strong and flexible. These long fibers or sh ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.