Protein - PBworks
... Protein is an energy supplying nutrient made up of carbon, hydrogen, oxygen and nitrogen. The nitrogen is what makes it different from carbohydrates and fats. Proteins are formed from the combining of 20 different amino acids into different combinations and patterns. There are at least 30,000 differ ...
... Protein is an energy supplying nutrient made up of carbon, hydrogen, oxygen and nitrogen. The nitrogen is what makes it different from carbohydrates and fats. Proteins are formed from the combining of 20 different amino acids into different combinations and patterns. There are at least 30,000 differ ...
Biochem 4 protein notes - The Bronx High School of Science
... NON-POLAR (aliphatic) ALA, VAL, ILE, LEU, PHE, TRP contain only hydrocarbons R groups = hydrophobicity ...
... NON-POLAR (aliphatic) ALA, VAL, ILE, LEU, PHE, TRP contain only hydrocarbons R groups = hydrophobicity ...
study guide - Dorman High School
... 1. Describe the four levels of protein structure. 2. Draw and describe the structure of a cell membrane, showing how phospholipids work to form the phospholipid bilayer. 3. Discuss why maintaining a normal pH is so critical in the human body and recognize acidosis and alkalosis. ...
... 1. Describe the four levels of protein structure. 2. Draw and describe the structure of a cell membrane, showing how phospholipids work to form the phospholipid bilayer. 3. Discuss why maintaining a normal pH is so critical in the human body and recognize acidosis and alkalosis. ...
Polypeptide Chain Synthesis: A Paper Simulation
... Involves two amino acids. Involves a dehydration synthesis. Involves a chemical reaction that occurs between two specific areas of the amino acid. Requires an –OH group and an –H from another –OH group ...
... Involves two amino acids. Involves a dehydration synthesis. Involves a chemical reaction that occurs between two specific areas of the amino acid. Requires an –OH group and an –H from another –OH group ...
Protein Metabolism
... or leucine favors rapid ubiquitination, whereas a stabilizing residue such as methionine or proline does not. – E3 enzymes are the readers of N-terminal residues. ...
... or leucine favors rapid ubiquitination, whereas a stabilizing residue such as methionine or proline does not. – E3 enzymes are the readers of N-terminal residues. ...
One Gene -One polypeptide
... Chapter 11.4 One gene=one polypeptide Overview of Protein Synthesis ...
... Chapter 11.4 One gene=one polypeptide Overview of Protein Synthesis ...
Chapter 11. Protein Structure and Function
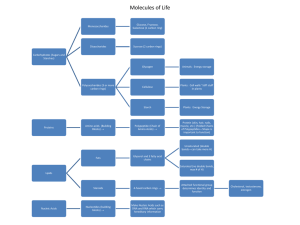
... • These are biopolymers that are constructed from a limited set of amino acids. • They are the most plentiful organic substances in the cell. • About half of the dry mass of a cell is composed of proteins. • They serve a wide range of functions. ...
... • These are biopolymers that are constructed from a limited set of amino acids. • They are the most plentiful organic substances in the cell. • About half of the dry mass of a cell is composed of proteins. • They serve a wide range of functions. ...
Chapter 2
... and regulate cell processes • Form bone and muscle • Transport substances into or out of cells • Help to fight disease ...
... and regulate cell processes • Form bone and muscle • Transport substances into or out of cells • Help to fight disease ...
Proteins
... structure are attracted to each other. The strongest bonds are made between cytosine amino acids. The sulphur atoms form a disulphide bridge at the amino acid cytosine. Bonds are also created by weak ionic and H bonds as well as hydrophobic ...
... structure are attracted to each other. The strongest bonds are made between cytosine amino acids. The sulphur atoms form a disulphide bridge at the amino acid cytosine. Bonds are also created by weak ionic and H bonds as well as hydrophobic ...
Protein Synthesis Review
... Proteins are polymers, made up of monomers called amino acids There are 20 different types of amino acids ...
... Proteins are polymers, made up of monomers called amino acids There are 20 different types of amino acids ...
Peptide Bonds - Newcastle University
... Two amino acids joined together are called a dipeptide. The condensation reaction you have just seen can repeat so any more amino acids can add together in the same way, forming a long chain called a polypeptide. For each protein, the order of amino acid residues is specific and different – it is th ...
... Two amino acids joined together are called a dipeptide. The condensation reaction you have just seen can repeat so any more amino acids can add together in the same way, forming a long chain called a polypeptide. For each protein, the order of amino acid residues is specific and different – it is th ...
How Did Life Begin? Unit Objectives Vocabulary: Miller
... By the end of this unit students will be able to: o Describe what it means to be alive using no less than six criteria. o List the two components of cell theory and explain how they apply to the fossil record explored in unit 1 and the origin of life itself. o Explain the origin of organic molecules ...
... By the end of this unit students will be able to: o Describe what it means to be alive using no less than six criteria. o List the two components of cell theory and explain how they apply to the fossil record explored in unit 1 and the origin of life itself. o Explain the origin of organic molecules ...
Proteins - RMC Science Home
... Proteins are groups of Amino Acids that are bonded together by a peptide bond. Contain hydrogen, oxygen, carbon and nitrogen The main function of proteins is to build and maintain tissues. Can also be used for energy but ONLY if carbohydrate and fat stores are depleated. ...
... Proteins are groups of Amino Acids that are bonded together by a peptide bond. Contain hydrogen, oxygen, carbon and nitrogen The main function of proteins is to build and maintain tissues. Can also be used for energy but ONLY if carbohydrate and fat stores are depleated. ...
Document
... Amino acids link by dehydration reaction (amino group towards carboxyl) Protein = hundreds to thousands of aminoacids – polypeptides Peptide units can rotate about carbon bonds –rotation angles restricted by repulsive forces when too closed together; overall rotation arrangement = conformation =>the ...
... Amino acids link by dehydration reaction (amino group towards carboxyl) Protein = hundreds to thousands of aminoacids – polypeptides Peptide units can rotate about carbon bonds –rotation angles restricted by repulsive forces when too closed together; overall rotation arrangement = conformation =>the ...
TABLE 3–1 Some Common Types of Enzymes
... general name used for enzymes that synthesize molecules in anabolic reactions by condensing two smaller molecules together. catalyze the rearrangement of bonds within a single molecule. catalyze polymerization reactions such as the synthesis of DNA and RNA. catalyze the addition of phosphate groups ...
... general name used for enzymes that synthesize molecules in anabolic reactions by condensing two smaller molecules together. catalyze the rearrangement of bonds within a single molecule. catalyze polymerization reactions such as the synthesis of DNA and RNA. catalyze the addition of phosphate groups ...
Catalogue Number CTK-611 Synonyms TFF
... TFF-2, Spasmolytic polypeptide, Spasmolysin, SML1, Trefoil factor 2, SP, TFF2. Proteins of the TFF family are characterized by obtaining a minimum of 1 copy of the trefoil motif, a 40-amino acid domain that contains 3 conserved disulfides. Trefoil Factors are stable secretory proteins expressed in g ...
... TFF-2, Spasmolytic polypeptide, Spasmolysin, SML1, Trefoil factor 2, SP, TFF2. Proteins of the TFF family are characterized by obtaining a minimum of 1 copy of the trefoil motif, a 40-amino acid domain that contains 3 conserved disulfides. Trefoil Factors are stable secretory proteins expressed in g ...
Covalent Reactions Atoms SHARE electrons
... Proteins= polymers of amino acid monomers Many functions! Some examples: • Keratin- skin and nails • Collagen- ligaments, tendons, skin • Many hormones • Actin and Myosin- allow muscles to contract • Hemoglobin- transport oxygen in blood • Antibodies in the blood • Allow movement through cell membra ...
... Proteins= polymers of amino acid monomers Many functions! Some examples: • Keratin- skin and nails • Collagen- ligaments, tendons, skin • Many hormones • Actin and Myosin- allow muscles to contract • Hemoglobin- transport oxygen in blood • Antibodies in the blood • Allow movement through cell membra ...
NAME
... 1. Check with the other groups in the class. What other variants of the gene exist? How similar or dissimilar were their DNA sequence? ...
... 1. Check with the other groups in the class. What other variants of the gene exist? How similar or dissimilar were their DNA sequence? ...
Rice Krispie Treats
... 1. Check with the other groups in the class. What other variants of the gene exist? How similar or dissimilar were their DNA sequence? ...
... 1. Check with the other groups in the class. What other variants of the gene exist? How similar or dissimilar were their DNA sequence? ...
Protein Purification
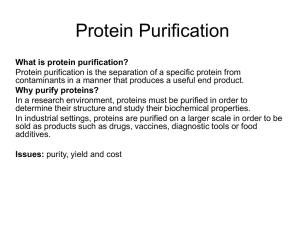
... • separates proteins based on size and shape. • The support for gel-filtration chromatography are beads which contain holes, called "pores," of given sizes. • Larger molecules, which can't penetrate the pores, move around the beads and migrate through the spaces which separate the beads faster than ...
... • separates proteins based on size and shape. • The support for gel-filtration chromatography are beads which contain holes, called "pores," of given sizes. • Larger molecules, which can't penetrate the pores, move around the beads and migrate through the spaces which separate the beads faster than ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.