Chem 464 Biochemistry
... two internal amino acids of the tetrapeptide cannot have ionizable R groups. 5. Pauling and Corey's studies of the peptide bond showed that: A) at pH 7, many different peptide bond conformations are equally probable. B) peptide bonds are essentially planar, with no rotation about the C-N axis. C) pe ...
... two internal amino acids of the tetrapeptide cannot have ionizable R groups. 5. Pauling and Corey's studies of the peptide bond showed that: A) at pH 7, many different peptide bond conformations are equally probable. B) peptide bonds are essentially planar, with no rotation about the C-N axis. C) pe ...
AB123Abstract - PSI AOAPO 2016 Conference
... National Institute of Plant Genome Research, Aruna Asaf Ali Marg, New Delhi 110067, India ...
... National Institute of Plant Genome Research, Aruna Asaf Ali Marg, New Delhi 110067, India ...
Protein Synthesis
... Step 2: Translation Location: in the cytoplasm, on the ribosome Purpose: to convert the instructions of RNA (order of bases) into amino acids, this will make up the protein. Events of translation: 1.) The first three bases of mRNA (codon) join the ribosome. AUG – is the start codon 2.) tRNA brings t ...
... Step 2: Translation Location: in the cytoplasm, on the ribosome Purpose: to convert the instructions of RNA (order of bases) into amino acids, this will make up the protein. Events of translation: 1.) The first three bases of mRNA (codon) join the ribosome. AUG – is the start codon 2.) tRNA brings t ...
Core Proteome
... produce glycans, attached to proteins, lipids or other organic molecules. Glycosylation is a form of co-translational and post-translational modification. Glycans serve as a variety of structural and functional roles in membrane and secreted proteins. It is an enzyme-directed site-specific pro ...
... produce glycans, attached to proteins, lipids or other organic molecules. Glycosylation is a form of co-translational and post-translational modification. Glycans serve as a variety of structural and functional roles in membrane and secreted proteins. It is an enzyme-directed site-specific pro ...
Protein Synthesis
... 1. Imagine that the following string of letters are nitrogen bases on one side of a DNA molecule. The DNA molecule has opened to this particular section of itself (called a gene) to allow a messenger RNA to be formed for the making of a protein that is responsible for golden blonde hair. DNA: T A C ...
... 1. Imagine that the following string of letters are nitrogen bases on one side of a DNA molecule. The DNA molecule has opened to this particular section of itself (called a gene) to allow a messenger RNA to be formed for the making of a protein that is responsible for golden blonde hair. DNA: T A C ...
Proteins are made of subunits called amino acids and are
... COLOR and LABEL the parts of a nucleotide --- sugar (5-sided)-green, phosphate group (round)yellow, and nitrogen base (6-sided)-blue. ATP used for cellular energy is a high energy nucleotide with three phosphate groups. Color code the ATP and LABEL THE PHOSPHATES. ...
... COLOR and LABEL the parts of a nucleotide --- sugar (5-sided)-green, phosphate group (round)yellow, and nitrogen base (6-sided)-blue. ATP used for cellular energy is a high energy nucleotide with three phosphate groups. Color code the ATP and LABEL THE PHOSPHATES. ...
Chapter 5
... • Polymers of AA – 20 AA, all varied in their “R” groups – 9 essential AA can not be made by the body ...
... • Polymers of AA – 20 AA, all varied in their “R” groups – 9 essential AA can not be made by the body ...
Topic 2.2: Proteins
... groups are always in the same position, therefore the bonding between Rgroups will always be the same, and the hydrophobic and hydrophillic interactions will always be the same and therefore the tertialy structure of a specific protein is always identical. ...
... groups are always in the same position, therefore the bonding between Rgroups will always be the same, and the hydrophobic and hydrophillic interactions will always be the same and therefore the tertialy structure of a specific protein is always identical. ...
g. ¶I - wwphs
... d.-Twists, bends, loops, and folds of a new polypeptide chain; hydrogen bonds between R groups make some stretches of amino acids coil, and other regions form sheets or ioops Comes in two slightly different forms, alpha and beta; two of each form make up one hemoglobin molecule in humans Airoteins t ...
... d.-Twists, bends, loops, and folds of a new polypeptide chain; hydrogen bonds between R groups make some stretches of amino acids coil, and other regions form sheets or ioops Comes in two slightly different forms, alpha and beta; two of each form make up one hemoglobin molecule in humans Airoteins t ...
מצגת של PowerPoint
... - first rank, most important Play central roles in all biological processes ...
... - first rank, most important Play central roles in all biological processes ...
File
... The next step in abiogenesis is the movement from monomers to polymers in order to make molecules that are capable of complex reactions or functions, like information storage for DNA, enzymatic activity for proteins, and energy storage with sugars. These polymers, along with the 4th macromolecule, l ...
... The next step in abiogenesis is the movement from monomers to polymers in order to make molecules that are capable of complex reactions or functions, like information storage for DNA, enzymatic activity for proteins, and energy storage with sugars. These polymers, along with the 4th macromolecule, l ...
Introduction to Protein Science Architecture, Function
... 3.Sensitive position in the active site Ex) Gln-> Arg substitution, Malate dehydrogenase -> ...
... 3.Sensitive position in the active site Ex) Gln-> Arg substitution, Malate dehydrogenase -> ...
Translation
... must then be translated into _____________. The processes of transcription and translation together are called _________________________. The process of transcription occurs in the ____________ of a cell. Translation occurs at the ______________, which can be found free in the cytoplasm or attached ...
... must then be translated into _____________. The processes of transcription and translation together are called _________________________. The process of transcription occurs in the ____________ of a cell. Translation occurs at the ______________, which can be found free in the cytoplasm or attached ...
Distinguishing cell types with masks
... Neurobiology as well as members from INSERM have developed a technique called fluorescent non-canonical amino acid tagging (FUNCAT) that allows visualization of changes in protein synthesis in the proteome on the time scale of minutes. “We know that some synaptic proteins can be synthesized only in ...
... Neurobiology as well as members from INSERM have developed a technique called fluorescent non-canonical amino acid tagging (FUNCAT) that allows visualization of changes in protein synthesis in the proteome on the time scale of minutes. “We know that some synaptic proteins can be synthesized only in ...
Combinatorial docking approach for structure prediction of large
... structure and the algorithm returns a list of possible structures ordered based on their probability. Similar structures are clustered together to avoid redundancy and a final list is made. An important note to make is that the program cannot predict whether proteins will combine and form an assembl ...
... structure and the algorithm returns a list of possible structures ordered based on their probability. Similar structures are clustered together to avoid redundancy and a final list is made. An important note to make is that the program cannot predict whether proteins will combine and form an assembl ...
Biochemistry Review Guide 2014
... Hydrophobic Tails At least one double bond between the carbons Cell membrane Hydrophilic Head Chemical Signals Nuts Boundary that surrounds cells ...
... Hydrophobic Tails At least one double bond between the carbons Cell membrane Hydrophilic Head Chemical Signals Nuts Boundary that surrounds cells ...
Enzymes/Macromolecules/Bonding
... Double sugar needs to be broken apart Only one enzyme can function for this reaction Shape of an Enzyme can determine its functions ...
... Double sugar needs to be broken apart Only one enzyme can function for this reaction Shape of an Enzyme can determine its functions ...
Chapter 5 – The Proteins and Amino Acids
... The Functions of Body Proteins The major role of dietary protein is to supply amino acids for the synthesis of proteins needed in the body, although dietary protein can also serve as an energy source. Proteins act as enzymes, as well as perform many other functions in the body. They help regulate wa ...
... The Functions of Body Proteins The major role of dietary protein is to supply amino acids for the synthesis of proteins needed in the body, although dietary protein can also serve as an energy source. Proteins act as enzymes, as well as perform many other functions in the body. They help regulate wa ...
Protein Structure and Folding
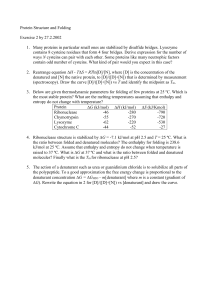
... Protein Structure and Folding Exercise 2 by 27.2.2002 1. Many proteins in particular small ones are stabilized by disulfide bridges. Lysozyme contains 8 cysteine residues that form 4 four bridges. Derive expression for the number of ways N cysteins can pair with each other. Some proteins like many n ...
... Protein Structure and Folding Exercise 2 by 27.2.2002 1. Many proteins in particular small ones are stabilized by disulfide bridges. Lysozyme contains 8 cysteine residues that form 4 four bridges. Derive expression for the number of ways N cysteins can pair with each other. Some proteins like many n ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.