Connections of Carbohydrate, Protein, and Lipid
... This hypothetical situation would have resulted in natural selection favoring those organisms that could exist by using the nutrients that remained in their environment and by manipulating these nutrients into materials upon which they could survive. Selection would favor those organisms that could ...
... This hypothetical situation would have resulted in natural selection favoring those organisms that could exist by using the nutrients that remained in their environment and by manipulating these nutrients into materials upon which they could survive. Selection would favor those organisms that could ...
H &
... The names and structures of some of the intermediate compounds in metabolism are complex. You do not need to memorize them, but they wi[ be used in the text to make it easierto followwhat is happening. Remembei also that all the steps ofthese reactions are catalyzedby enzyrnes. The phosphoryl group ...
... The names and structures of some of the intermediate compounds in metabolism are complex. You do not need to memorize them, but they wi[ be used in the text to make it easierto followwhat is happening. Remembei also that all the steps ofthese reactions are catalyzedby enzyrnes. The phosphoryl group ...
Document
... all have the same chemical formula: C12H22O11 they are ISOMERS of each other they can only be absorbed into the intestine & blood stream when they are broken down into their monomers (monosaccharides) by enzymes Examples ...
... all have the same chemical formula: C12H22O11 they are ISOMERS of each other they can only be absorbed into the intestine & blood stream when they are broken down into their monomers (monosaccharides) by enzymes Examples ...
Cellular Functions PP
... The protons then diffuse through a special proton channels called ATP synthase, down the concentration gradient back into the matrix of the mitochondria, creating ATP in the process. Chemiosmosis is the coupling of the protonmotive force and ATP synthesis. The final electron acceptor is Oxygen which ...
... The protons then diffuse through a special proton channels called ATP synthase, down the concentration gradient back into the matrix of the mitochondria, creating ATP in the process. Chemiosmosis is the coupling of the protonmotive force and ATP synthesis. The final electron acceptor is Oxygen which ...
Mitochondrial Energy Metabolism:
... by release of glucose from the liver (glycogen) • As we run out of glycogen we maintain the blood glucose level by making glucose from amino acids (protein) and other compounds • The energy to make glucose comes from burning fatty acids, which also generates ketones that are used as an alternative ...
... by release of glucose from the liver (glycogen) • As we run out of glycogen we maintain the blood glucose level by making glucose from amino acids (protein) and other compounds • The energy to make glucose comes from burning fatty acids, which also generates ketones that are used as an alternative ...
Rearrange the sentences into the correct sequence
... to glucose so that more glucose enters cells (e.g. muscle cells, but not liver cells) ...
... to glucose so that more glucose enters cells (e.g. muscle cells, but not liver cells) ...
Building Macromolecules Notes
... Glucose and Fructose both have the formula C6H12O6, Sometimes compounds may have the same formula, however they have different structures/ arrangements. In such cases, those compounds are called isomers. ...
... Glucose and Fructose both have the formula C6H12O6, Sometimes compounds may have the same formula, however they have different structures/ arrangements. In such cases, those compounds are called isomers. ...
gluconeogenesis
... In mammals, some tissues depend almost completely on glucose for their metabolic energy The brain alone requires about 120 g of glucose each day—more than half of all the glucose stored as glycogen in muscle and liver. However, the supply of glucose from these stores is not always sufficient; betwe ...
... In mammals, some tissues depend almost completely on glucose for their metabolic energy The brain alone requires about 120 g of glucose each day—more than half of all the glucose stored as glycogen in muscle and liver. However, the supply of glucose from these stores is not always sufficient; betwe ...
Connections of Carbohydrate, Protein, and Lipid
... muscle. The glycogen will be hydrolyzed into glucose monomers (G-1-P) if blood sugar levels drop. The presence of glycogen as a source of glucose allows ATP to be produced for a longer period of time during exercise. Glycogen is broken down into G-1-P and converted into G-6-P in both muscle and live ...
... muscle. The glycogen will be hydrolyzed into glucose monomers (G-1-P) if blood sugar levels drop. The presence of glycogen as a source of glucose allows ATP to be produced for a longer period of time during exercise. Glycogen is broken down into G-1-P and converted into G-6-P in both muscle and live ...
Macromolecules - Mr. Holmes` Biology
... • We begin with carbohydrates… • Carbohydrates are sugars we eat on a daily basis • Source of quick energy for our body • Carbohydrates are ALWAYS found in the Ratio of : 1 Carbon to 2 Hydrogen to 1 Oxygen = 1:2:1 • Remember this shape? • It is a carbohydrate monomer called glucose Glucose= C6H12O6 ...
... • We begin with carbohydrates… • Carbohydrates are sugars we eat on a daily basis • Source of quick energy for our body • Carbohydrates are ALWAYS found in the Ratio of : 1 Carbon to 2 Hydrogen to 1 Oxygen = 1:2:1 • Remember this shape? • It is a carbohydrate monomer called glucose Glucose= C6H12O6 ...
Cori Cycle - COFFEE BREAK CORNER
... It is the conversion of glucose into lactate in peripheral tissues, followed by conversion of lactate into glucose in liver From glycolysis especially in RBCs due to absence of mitochondria and muscle ...
... It is the conversion of glucose into lactate in peripheral tissues, followed by conversion of lactate into glucose in liver From glycolysis especially in RBCs due to absence of mitochondria and muscle ...
Pertubation of metabolism in IDD Q3-5 Joe - PBL-J-2015
... 3. Indicate the mechanisms by which beta ketoacid production is increased in response to oversupply of fatty acid to the liver, including the steps of the pathways of fatty acid metabolism that are up regulated The oversupply of fatty acids in the liver cells (in the absence of insulin) leads to the ...
... 3. Indicate the mechanisms by which beta ketoacid production is increased in response to oversupply of fatty acid to the liver, including the steps of the pathways of fatty acid metabolism that are up regulated The oversupply of fatty acids in the liver cells (in the absence of insulin) leads to the ...
Slide 1
... GLUCONEOGENESIS Gluconeogenesis is the synthesis of glucose from glucogenic precursors which are not of carbohydrate origin (gluconeogenic precursors) It occurs during prolonged fasting to synthesize glucose for tissues requiring continuous supply of glucose as a source of energy: Brain, RBCs, Kidn ...
... GLUCONEOGENESIS Gluconeogenesis is the synthesis of glucose from glucogenic precursors which are not of carbohydrate origin (gluconeogenic precursors) It occurs during prolonged fasting to synthesize glucose for tissues requiring continuous supply of glucose as a source of energy: Brain, RBCs, Kidn ...
Lecture: Biochemistry
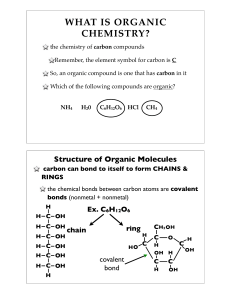
... A. Characteristics of Organic (Carbon containing) Compounds 1. Exceptions: CO (carbon monoxide) C02 (carbon dioxide) C (graphite and diamond) 2. carbon forms 4 covalent bonds (not ions) 3. carbon is relatively electroneutral (not e-neg) 4. carbon easily forms bonds with H, O, N 5. carbon can form si ...
... A. Characteristics of Organic (Carbon containing) Compounds 1. Exceptions: CO (carbon monoxide) C02 (carbon dioxide) C (graphite and diamond) 2. carbon forms 4 covalent bonds (not ions) 3. carbon is relatively electroneutral (not e-neg) 4. carbon easily forms bonds with H, O, N 5. carbon can form si ...
(3-D Molecules (key))
... atoms of each element in one sucrose molecule? 12 carbon, 22 hydrogen, 11 oxygen C12H22O11 b. Glucose is a monosaccharide; it is one simple sugar molecule based on a ring of carbon atoms. How many carbon rings do you see in a sucrose molecule? What do we call this kind of sugar? ...
... atoms of each element in one sucrose molecule? 12 carbon, 22 hydrogen, 11 oxygen C12H22O11 b. Glucose is a monosaccharide; it is one simple sugar molecule based on a ring of carbon atoms. How many carbon rings do you see in a sucrose molecule? What do we call this kind of sugar? ...
classsssssss
... male who suffers from periodic hemolysis demonstrate a low activity of glucose-6-phosphate dehydrogenase. Deficiency of which of the following erythrocyte enzymes has the same pathophysiology as this patient’s condition? • A. bisphosphoglycerate mutase • B. pyruvate kinase • C. hexokinase • D. trans ...
... male who suffers from periodic hemolysis demonstrate a low activity of glucose-6-phosphate dehydrogenase. Deficiency of which of the following erythrocyte enzymes has the same pathophysiology as this patient’s condition? • A. bisphosphoglycerate mutase • B. pyruvate kinase • C. hexokinase • D. trans ...
Slide 1
... – Energy yield depends on length of carbon chain (ex. 16C palmitic acid results in 129 ATPs, ~3.5x more than glucose) – Ketoacidosis: results if oxaloacetate in short supply; acetyl-CoA converted into ketones, which are weak acids; can occur due to starvation, low-carbohydrate diet, or by uncontroll ...
... – Energy yield depends on length of carbon chain (ex. 16C palmitic acid results in 129 ATPs, ~3.5x more than glucose) – Ketoacidosis: results if oxaloacetate in short supply; acetyl-CoA converted into ketones, which are weak acids; can occur due to starvation, low-carbohydrate diet, or by uncontroll ...
Lecture: Biochemistry I. Inorganic Compounds A. Water (H2O)
... i. antibodies - attach to foreign molecules ii. complement proteins - enhance response 4. Enzymes and Enzyme Function a. enzyme - a protein that catalyzes a reaction i. increase the rate of a natural reaction b. cofactor or coenzyme - essential for function i. could be a metal like Fe, Cu, Zn ii. ma ...
... i. antibodies - attach to foreign molecules ii. complement proteins - enhance response 4. Enzymes and Enzyme Function a. enzyme - a protein that catalyzes a reaction i. increase the rate of a natural reaction b. cofactor or coenzyme - essential for function i. could be a metal like Fe, Cu, Zn ii. ma ...
MCQs in Carbohydrate Metabolism
... (c) Ovum (d) Red cell 2. In aerobic glycolysis, glucose is first broken down to pyruvate and then to CO2 and H2O in the Kreb's cycle; but in anaerobic glycolysis it does not stop at pyruvate but forms lactate. Why? (a) Because pyruvate is toxic in larger concentration. (b) Because pyruvate can form ...
... (c) Ovum (d) Red cell 2. In aerobic glycolysis, glucose is first broken down to pyruvate and then to CO2 and H2O in the Kreb's cycle; but in anaerobic glycolysis it does not stop at pyruvate but forms lactate. Why? (a) Because pyruvate is toxic in larger concentration. (b) Because pyruvate can form ...
Enzymes & Energy
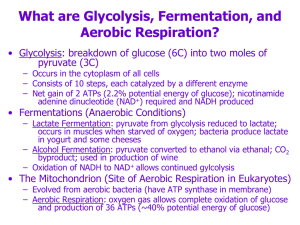
... Most dietary carbohydrate is burned as fuel within a few hours of absorption Three monosaccharides are absorbed from digested food - glucose, galactose, and fructose, but the last two are quickly converted to glucose All oxidative carbohydrate consumption is essentially a matter of glucose catab ...
... Most dietary carbohydrate is burned as fuel within a few hours of absorption Three monosaccharides are absorbed from digested food - glucose, galactose, and fructose, but the last two are quickly converted to glucose All oxidative carbohydrate consumption is essentially a matter of glucose catab ...
Slide 1
... As glycogen stores decrease, adipose triacylglycerols (TAGs)are also degraded, providing fatty acids as an alternative fuel and glycerol for the synthesis of glucose by gluconeogenesis. Amino acids are also released from the muscle to serve as gluconeogenic precursors. During an overnight fast, bloo ...
... As glycogen stores decrease, adipose triacylglycerols (TAGs)are also degraded, providing fatty acids as an alternative fuel and glycerol for the synthesis of glucose by gluconeogenesis. Amino acids are also released from the muscle to serve as gluconeogenic precursors. During an overnight fast, bloo ...
Chemistry 221 - Oregon State chemistry
... [Glucose is the unit from which starch, cellulose and glycogen are made up. Glucose is a ready source of energy. It is oxidized (combusted) to produce carbon dioxide and water, releasing energy in the process. However, unlike other hydrocarbon fuels, which are insoluble in water, the numerous OH gro ...
... [Glucose is the unit from which starch, cellulose and glycogen are made up. Glucose is a ready source of energy. It is oxidized (combusted) to produce carbon dioxide and water, releasing energy in the process. However, unlike other hydrocarbon fuels, which are insoluble in water, the numerous OH gro ...
BHS 150.2 Biochemistry Date: 02/08/13, 1st hour Notetaker: Laurel
... Q1: Receptor mechanism of action for glucagon and insulin. Know mechanisms for final exam. Q2: Think about glycogen synthetase, glycogen phosphatase, pyruvate kinase, and the effects of high levels of insulin. Insulin activates a phosphatase, which removes a phosphate group. Activates things to stor ...
... Q1: Receptor mechanism of action for glucagon and insulin. Know mechanisms for final exam. Q2: Think about glycogen synthetase, glycogen phosphatase, pyruvate kinase, and the effects of high levels of insulin. Insulin activates a phosphatase, which removes a phosphate group. Activates things to stor ...
This is Most of an Old Exam
... Cellular oxidation of food fuels is the immediate source of electrons for oxidative phosphorylation. B. In oxidative phosphorylation, both the electron transport proteins and the ATP synthase molecules are in the same membrane. C. NAD+ and FAD+ are hydrogen carrier molecules. NAD+ can carry one hydr ...
... Cellular oxidation of food fuels is the immediate source of electrons for oxidative phosphorylation. B. In oxidative phosphorylation, both the electron transport proteins and the ATP synthase molecules are in the same membrane. C. NAD+ and FAD+ are hydrogen carrier molecules. NAD+ can carry one hydr ...
Glucose

Glucose is a sugar with the molecular formula C6H12O6. The name ""glucose"" (/ˈɡluːkoʊs/) comes from the Greek word γλευκος, meaning ""sweet wine, must"". The suffix ""-ose"" is a chemical classifier, denoting a carbohydrate. It is also known as dextrose or grape sugar. With 6 carbon atoms, it is classed as a hexose, a sub-category of monosaccharides. α-D-glucose is one of the 16 aldose stereoisomers. The D-isomer (D-glucose) occurs widely in nature, but the L-isomer (L-glucose) does not. Glucose is made during photosynthesis from water and carbon dioxide, using energy from sunlight. The reverse of the photosynthesis reaction, which releases this energy, is a very important source of power for cellular respiration. Glucose is stored as a polymer, in plants as starch and in animals as glycogen.