- Wiley Online Library
... nickel, iridium, osmium, copper, platinum and titanium. Despite the variation of these parameters, the range of functional groups that can be reduced by metal borohydrides is very limited. We now report a method which makes it possible also to reduce amino acids, carboxylic acids, amides, nitriles, ...
... nickel, iridium, osmium, copper, platinum and titanium. Despite the variation of these parameters, the range of functional groups that can be reduced by metal borohydrides is very limited. We now report a method which makes it possible also to reduce amino acids, carboxylic acids, amides, nitriles, ...
Williamson Ether Synthesis
... Ethers are prepared by SN2 reactions. Ethers can be prepared by the reaction of an alkoxide with a primary haloalkane or sulfonate ester under SN2 conditions. The parent alcohol of the alkoxide can be used as the solvent, however other polar solvents are often better, such as DMSO (dimethyl sulfoxid ...
... Ethers are prepared by SN2 reactions. Ethers can be prepared by the reaction of an alkoxide with a primary haloalkane or sulfonate ester under SN2 conditions. The parent alcohol of the alkoxide can be used as the solvent, however other polar solvents are often better, such as DMSO (dimethyl sulfoxid ...
Syn Addition
... Note that we still have an acidic hydrogen and, thus, can react with another alkyl group in this way to make RCCR’ Alkyl halides can be obtained from alcohols ...
... Note that we still have an acidic hydrogen and, thus, can react with another alkyl group in this way to make RCCR’ Alkyl halides can be obtained from alcohols ...
Organic Reactions
... a. Alkene + halogen gas 1,2-dihaloalkane b. Diatomic gas has two atoms – both add to opposite sides of the double bond (and opposite sides of the molecule) c. Uses: Chlorine + ethane 1,2-dichloroethane: used as starting material for PVC d. Uses: Br2 dissolved in dichloromethane is used to distin ...
... a. Alkene + halogen gas 1,2-dihaloalkane b. Diatomic gas has two atoms – both add to opposite sides of the double bond (and opposite sides of the molecule) c. Uses: Chlorine + ethane 1,2-dichloroethane: used as starting material for PVC d. Uses: Br2 dissolved in dichloromethane is used to distin ...
Slides
... t C-Acylation leads directly to β -diketones l N-acylated products can be formed, but they are unstable and ...
... t C-Acylation leads directly to β -diketones l N-acylated products can be formed, but they are unstable and ...
CHM230 OXIDATION OF CYCLOHEXANOL TO CYCLOHEXANONE
... Add 20 - 30 ml of water to the round bottom and distill about 30 ml of distillate into a graduated cylinder. The distillate should be a mixture of cyclohexanone and water that contains excess acetic acid. Transfer the distillate to a separatory funnel or beaker. 6. Add 3.5 grams of sodium carbonate ...
... Add 20 - 30 ml of water to the round bottom and distill about 30 ml of distillate into a graduated cylinder. The distillate should be a mixture of cyclohexanone and water that contains excess acetic acid. Transfer the distillate to a separatory funnel or beaker. 6. Add 3.5 grams of sodium carbonate ...
Microsoft Word
... Several studies were carried out to achieve selective formation of 2-6AMN. Acylation of 2-methoxy naphthalene with acetic anhydride using unsupported phosphotungstic acid as catalyst proceeds efficiently but gives the unwanted 1,6-isomer as the major product with >98% selectivity. To choose appropri ...
... Several studies were carried out to achieve selective formation of 2-6AMN. Acylation of 2-methoxy naphthalene with acetic anhydride using unsupported phosphotungstic acid as catalyst proceeds efficiently but gives the unwanted 1,6-isomer as the major product with >98% selectivity. To choose appropri ...
Acid-Catalyzed Dehydration of Alcohols
... as a layer on the surface of the acid-alcohol mixture (the density of the alkene is less than one); the gas can then be collected over water or the liquid layer can be removed by simple distillation to give the final alkene product. In alcohols where there are more than two kinks of -hydrogens, the ...
... as a layer on the surface of the acid-alcohol mixture (the density of the alkene is less than one); the gas can then be collected over water or the liquid layer can be removed by simple distillation to give the final alkene product. In alcohols where there are more than two kinks of -hydrogens, the ...
Aldehydes and Ketones
... Aldehyde are usually more reactive toward nucleophilic substitutions than ketones because of both steric and electronic effects. In aldehydes, the relatively small hydrogen atom is attached to one side of the carbonyl group. While a larger ...
... Aldehyde are usually more reactive toward nucleophilic substitutions than ketones because of both steric and electronic effects. In aldehydes, the relatively small hydrogen atom is attached to one side of the carbonyl group. While a larger ...
Nucleophilic Addition: The Grignard reagent
... one considers the electronegativities of the elements bonded to the carbon, it is clear that the C atom in the product is more electron rich than the C atom in the reactant. ...
... one considers the electronegativities of the elements bonded to the carbon, it is clear that the C atom in the product is more electron rich than the C atom in the reactant. ...
File
... Treatment of the ionic rhodium complex [Rh(COD)2][BF4] (COD = cyclo-octa-1,4-diene) with bis(diphenylphosphino)ethane (‘dppe’) in n-butanol leads to the formation of a highlyeffective system for the hydrogenation of alkenes (a) Draw a catalytic cycle for the conversion of ethene and hydrogen to eth ...
... Treatment of the ionic rhodium complex [Rh(COD)2][BF4] (COD = cyclo-octa-1,4-diene) with bis(diphenylphosphino)ethane (‘dppe’) in n-butanol leads to the formation of a highlyeffective system for the hydrogenation of alkenes (a) Draw a catalytic cycle for the conversion of ethene and hydrogen to eth ...
Organic Synthesis Part 2
... b) sodium borohydrides the parent compound is NaBH4 (sodium borohydride) which is available as a white powder. It is only moderately reactive towards protic solvents (H2O>MeOH>EtOH) and is usually used in ethanolic solution. It is less reactive than LAH, for example it reacts only slowly with esters ...
... b) sodium borohydrides the parent compound is NaBH4 (sodium borohydride) which is available as a white powder. It is only moderately reactive towards protic solvents (H2O>MeOH>EtOH) and is usually used in ethanolic solution. It is less reactive than LAH, for example it reacts only slowly with esters ...
replacing the - Shasha iSeminar
... There are also side reactions involving the POCl3 reacting with the alcohol. Other reactions involving phosphorus halides Instead of using phosphorus(III) bromide or iodide, the alcohol is usually heated under reflux with a mixture of red phosphorus and either bromine or iodine. The phosphorus first ...
... There are also side reactions involving the POCl3 reacting with the alcohol. Other reactions involving phosphorus halides Instead of using phosphorus(III) bromide or iodide, the alcohol is usually heated under reflux with a mixture of red phosphorus and either bromine or iodine. The phosphorus first ...
Nonracemic Allylic Boronates through Enantiotopic-Group
... lower selectivity were observed in the cross-coupling with 1bromo-2-methylpropene (18% 1H NMR yield, 85:15 er for the former; 13% 1H NMR yield, 89:11 er for the latter). These experiments suggest that the active participant in the crosscoupling is less likely to be the bis(boronic acid)-derived from ...
... lower selectivity were observed in the cross-coupling with 1bromo-2-methylpropene (18% 1H NMR yield, 85:15 er for the former; 13% 1H NMR yield, 89:11 er for the latter). These experiments suggest that the active participant in the crosscoupling is less likely to be the bis(boronic acid)-derived from ...
A Model for Catalytically Active Zinc(I1) Ion in Liver
... Abstract: The role of Zn" ion at the active center of liver alcohol dehydrogenase has been well-defined for the first time by the comparative studies of Zn"[ 12]aneN3, 1 ([12]aneN3 = 1,5,9-triazacyclododecane,L,),Zn"[ 12]aneN4, 2 ([ 12]aneN4 = 1,4,7,10-tetraazacyclododecane,L2),Zn"[ 14]aneN4, 3 ([ 1 ...
... Abstract: The role of Zn" ion at the active center of liver alcohol dehydrogenase has been well-defined for the first time by the comparative studies of Zn"[ 12]aneN3, 1 ([12]aneN3 = 1,5,9-triazacyclododecane,L,),Zn"[ 12]aneN4, 2 ([ 12]aneN4 = 1,4,7,10-tetraazacyclododecane,L2),Zn"[ 14]aneN4, 3 ([ 1 ...
PAKISTAN SHIPOWNERS` GOVERNMENT COLLEGE,
... (vi) a) What is Water Gas? Give two method of its preparation. b) Write Auto-Oxidation and Reduction of Chlorine. (vii) a) Write a note on complex Hydrides. b) Write the Chemical formula of the following.(Any four) ...
... (vi) a) What is Water Gas? Give two method of its preparation. b) Write Auto-Oxidation and Reduction of Chlorine. (vii) a) Write a note on complex Hydrides. b) Write the Chemical formula of the following.(Any four) ...
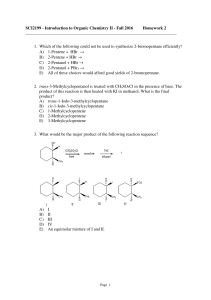
SCI2199 - Introduction to Organic Chemistry II
... A) an SN1-type reaction involving the protonated alcohol as the substrate. B) an SN2-type reaction involving the protonated alcohol as the substrate. C) an E1-type reaction involving the protonated alcohol as the substrate. D) an E2-type reaction involving the protonated alcohol as the substrate. E) ...
... A) an SN1-type reaction involving the protonated alcohol as the substrate. B) an SN2-type reaction involving the protonated alcohol as the substrate. C) an E1-type reaction involving the protonated alcohol as the substrate. D) an E2-type reaction involving the protonated alcohol as the substrate. E) ...
molecules Palladium and Organocatalysis: An Excellent Recipe for Asymmetric Synthesis
... activation, enamine activation, and palladium-catalyzed enyne cycloisomerization [46]. Córdova’s methodology allows the preparation of functionalized optically active cyclopentenes with all-carbon quaternary stereocenters in good diastereo- and high enantioselectivities from simple starting material ...
... activation, enamine activation, and palladium-catalyzed enyne cycloisomerization [46]. Córdova’s methodology allows the preparation of functionalized optically active cyclopentenes with all-carbon quaternary stereocenters in good diastereo- and high enantioselectivities from simple starting material ...
chapter 8 lecture
... • Recall that when alkyl halides have two or more different carbons, more than one alkene product is formed. ...
... • Recall that when alkyl halides have two or more different carbons, more than one alkene product is formed. ...
heptane
... 8. Are the following pairs enantiomers or diastereomers? Also label each molecule as a D sugar or L sugar. ...
... 8. Are the following pairs enantiomers or diastereomers? Also label each molecule as a D sugar or L sugar. ...
Baylis–Hillman reaction

The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.DABCO is one of the most frequently used tertiary amine catalysts for this reaction. In addition, nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.MBH reaction has several advantages as a useful synthetic method: 1) It is an atom-economic coupling of easily prepared starting materials. 2) Reaction of a pro-chiral electrophile generates a chiral center, therefore an asymmetric synthesis is possible. 3) Reaction products usually contain multiple functionalities in a proximity so that a variety of further transformations are possible. 4) It can employ a nucleophilic organo-catalytic system without the use of heavy metal under mild conditions.Several reviews have been written.