Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions
... Primary amine adds to C=O Proton is lost from N and adds to O to yield an amino alcohol (carbinolamine) Protonation of OH converts it into water as the leaving group Result is iminium ion, which loses proton Acid is required for loss of OH– too much acid blocks RNH2 ...
... Primary amine adds to C=O Proton is lost from N and adds to O to yield an amino alcohol (carbinolamine) Protonation of OH converts it into water as the leaving group Result is iminium ion, which loses proton Acid is required for loss of OH– too much acid blocks RNH2 ...
7. Alkenes: Reactions and Synthesis
... Hydroxylation and Cleavage Hydroxylation adds OH to each end of C=C Stereochemistry of addition is syn Product is a 1,2-dialcohol or diol (also called a glycol) ...
... Hydroxylation and Cleavage Hydroxylation adds OH to each end of C=C Stereochemistry of addition is syn Product is a 1,2-dialcohol or diol (also called a glycol) ...
The first practical method for asymmetric epoxidation
... identical reaction conditions. However, for the less-reactive substrates in Table I (5a, 6a, and 7a), the “1-equiv” conditions described above were necessary to achieve reasonable reaction rates. Even under the “1-equiv” conditions, allylic alcohol 7a required almost 2 days to approach completion. F ...
... identical reaction conditions. However, for the less-reactive substrates in Table I (5a, 6a, and 7a), the “1-equiv” conditions described above were necessary to achieve reasonable reaction rates. Even under the “1-equiv” conditions, allylic alcohol 7a required almost 2 days to approach completion. F ...
Answers
... Identify the acetal and hemiacetal groups in a molecule Explain the mechanism of acetal formation Predict the products of acetal formation given starting materials Given an acetal, do a retrosynthesis to the corresponding carbonyl and alcohols or diol Be able to compare/contrast acetal for ...
... Identify the acetal and hemiacetal groups in a molecule Explain the mechanism of acetal formation Predict the products of acetal formation given starting materials Given an acetal, do a retrosynthesis to the corresponding carbonyl and alcohols or diol Be able to compare/contrast acetal for ...
Oxidation of alcohol to carboxylic acid under mild acidic condition
... biphenyl-4-carboxylate(1) with sodium per iodide/sodium bromide and TEMPO as a catalyst gives (3aR,4R,5R,6aS)-5-[(biphenyl-4-ylcarbonyl)oxy]-2-oxohexahydro-2H-cyclopenta[b] furan -4-carboxylic acid (2), which on reaction with different alcohols in presence of EDC.HCl/DMAP gives corresponding esters ...
... biphenyl-4-carboxylate(1) with sodium per iodide/sodium bromide and TEMPO as a catalyst gives (3aR,4R,5R,6aS)-5-[(biphenyl-4-ylcarbonyl)oxy]-2-oxohexahydro-2H-cyclopenta[b] furan -4-carboxylic acid (2), which on reaction with different alcohols in presence of EDC.HCl/DMAP gives corresponding esters ...
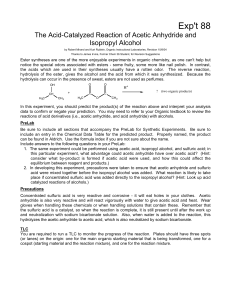
The Acid-Catalyzed Reaction of Acetic
... consider what by-product is formed if acetic acid were used, and how this could affect the equilibrium between reagent and products.) 2. In developing this experiment, precautions were taken to ensure that acetic anhydride and sulfuric acid were mixed together before the isopropyl alcohol was added. ...
... consider what by-product is formed if acetic acid were used, and how this could affect the equilibrium between reagent and products.) 2. In developing this experiment, precautions were taken to ensure that acetic anhydride and sulfuric acid were mixed together before the isopropyl alcohol was added. ...
Bifunctional Asymmetric Catalysis: Cooperative Lewis Acid/Base
... to be the best cocatalyst across the board; ironically, indiumbased Lewis acids have infrequently been used in organic synthesis, and their coordination chemistry is not well-explored.17 One reason may be that InIII binds to many ligands reversibly and with comparatively low affinity.18 The most str ...
... to be the best cocatalyst across the board; ironically, indiumbased Lewis acids have infrequently been used in organic synthesis, and their coordination chemistry is not well-explored.17 One reason may be that InIII binds to many ligands reversibly and with comparatively low affinity.18 The most str ...
Cooperative Lewis Acid/Base Systems
... to be the best cocatalyst across the board; ironically, indiumbased Lewis acids have infrequently been used in organic synthesis, and their coordination chemistry is not well-explored.17 One reason may be that InIII binds to many ligands reversibly and with comparatively low affinity.18 The most str ...
... to be the best cocatalyst across the board; ironically, indiumbased Lewis acids have infrequently been used in organic synthesis, and their coordination chemistry is not well-explored.17 One reason may be that InIII binds to many ligands reversibly and with comparatively low affinity.18 The most str ...
Experiment 7-Reduction
... A reduction is often defined as the gain of two hydrogen atoms or the loss of an oxygen atom, or both. This leads to a very important conversion reaction, where aldehydes and ketones are reduced to primary and secondary alcohols. O ...
... A reduction is often defined as the gain of two hydrogen atoms or the loss of an oxygen atom, or both. This leads to a very important conversion reaction, where aldehydes and ketones are reduced to primary and secondary alcohols. O ...
Rhenium(VII) Catalysis of Prins Cyclization Reactions
... All three aldehydes worked well in the reaction, although the more complex aldehyde 17, prepared by a metathesis reaction between crotonaldehyde and the corresponding terminal alkene, was noticeably slower than the others. All of the products showed very good selectivity for the equatorial alcohol T ...
... All three aldehydes worked well in the reaction, although the more complex aldehyde 17, prepared by a metathesis reaction between crotonaldehyde and the corresponding terminal alkene, was noticeably slower than the others. All of the products showed very good selectivity for the equatorial alcohol T ...
Lecture #
... Conjugate reductions and selectivity Hard vs. Soft nucleophiles and electrophiles: understanding polarizability by considering electronegativity, electron screening, and delocalization Other nucleophiles for conjugate additions: organocuprates, thiols Conjugate additions of enolates: Michael reactio ...
... Conjugate reductions and selectivity Hard vs. Soft nucleophiles and electrophiles: understanding polarizability by considering electronegativity, electron screening, and delocalization Other nucleophiles for conjugate additions: organocuprates, thiols Conjugate additions of enolates: Michael reactio ...
Aldehydes and ketones
... • Tautomers are isomers which differ in the placement of: – A hydrogen atom – A double bond – The keto form has a C=O while the enol form has a C=C. ...
... • Tautomers are isomers which differ in the placement of: – A hydrogen atom – A double bond – The keto form has a C=O while the enol form has a C=C. ...
Carbonyl The carbonyl function, C=O, exists in a number of organic
... groups to form alcohols constitute a major process in organic chemical synthesis. These reagents add easily to the carbonyl group but as strong bases they can also form alphacarbanions that give aldol reactions. The aldol reactions are minimized by working at cold temperatures. The reaction is exemp ...
... groups to form alcohols constitute a major process in organic chemical synthesis. These reagents add easily to the carbonyl group but as strong bases they can also form alphacarbanions that give aldol reactions. The aldol reactions are minimized by working at cold temperatures. The reaction is exemp ...
Development of Novel Catalytic Asymmetric Reactions using
... encouraged by the cooperative action presented in Scheme 9, we also investigated a Mannich-type reaction employing imine, which exhibits high affinity to protic acid, as the electrophilic agent. We found that the aqua complex 1 was effective in promoting Mannich-type reactions with various imines an ...
... encouraged by the cooperative action presented in Scheme 9, we also investigated a Mannich-type reaction employing imine, which exhibits high affinity to protic acid, as the electrophilic agent. We found that the aqua complex 1 was effective in promoting Mannich-type reactions with various imines an ...
Chapter 14 Selenium reagents
... oxide, benzeneseleninic acid (in combination with hydrogen peroxide) and benzeneseleninic anhydride. ...
... oxide, benzeneseleninic acid (in combination with hydrogen peroxide) and benzeneseleninic anhydride. ...
Lesson 14.1 Acid
... The Bronsted-Lowry concept of acids and bases has a greater scope than the Arrhenius concept. In the Bronsted concept: 1. A base is a species that accepts protons (Thus, OH- is only one example of a base). 2. Acids and bases can be ions as well as molecules. 3. Acid-base reactions are not restricte ...
... The Bronsted-Lowry concept of acids and bases has a greater scope than the Arrhenius concept. In the Bronsted concept: 1. A base is a species that accepts protons (Thus, OH- is only one example of a base). 2. Acids and bases can be ions as well as molecules. 3. Acid-base reactions are not restricte ...
chemistry 232 elementary organic chemistry ii
... Acid-Base Protonation/Deprotonation Reactions (Ch. 7 & 10) Protonation/Deprotonation of Alcohols Deprotonation of Alkynes Acid-Catalyzed Rearrangements (Ch. 9 & 10) via SN1 Reaction Pathway (step-wise) The Pinacol Rearrangement Nucleophilic Addition to Carbonyl Compounds (Ch. 15, 16, & 17) Organomet ...
... Acid-Base Protonation/Deprotonation Reactions (Ch. 7 & 10) Protonation/Deprotonation of Alcohols Deprotonation of Alkynes Acid-Catalyzed Rearrangements (Ch. 9 & 10) via SN1 Reaction Pathway (step-wise) The Pinacol Rearrangement Nucleophilic Addition to Carbonyl Compounds (Ch. 15, 16, & 17) Organomet ...
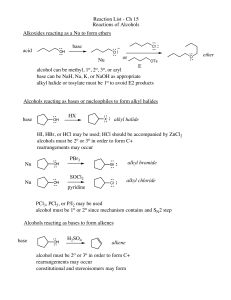
alcohols, alkyl halides, and nucleophilic substitutions
... separately. What structural change correlates with reactivity? Assuming this to be an SN1 reaction (see scheme in part B), and that the reaction is faster for more stable cation intermediates, indicate the order of stability of all the carbon cations formed from each alcohol. Examine the cations and ...
... separately. What structural change correlates with reactivity? Assuming this to be an SN1 reaction (see scheme in part B), and that the reaction is faster for more stable cation intermediates, indicate the order of stability of all the carbon cations formed from each alcohol. Examine the cations and ...
Additional file 1
... 1-Acenaphthen-5-yl-ethanone (3): Pyridinium dichromate (3.0 g, 9.0 mmol) was added to a stirred solution of alcohol 2 (1.8 g, 9.0 mmol) and powdered 4Å molecular sieve (0.75 g) in anhydrous CH2Cl2 (50 mL) at 0°C. After the addition was complete, the mixture was stirred at room temperature for 2 h, t ...
... 1-Acenaphthen-5-yl-ethanone (3): Pyridinium dichromate (3.0 g, 9.0 mmol) was added to a stirred solution of alcohol 2 (1.8 g, 9.0 mmol) and powdered 4Å molecular sieve (0.75 g) in anhydrous CH2Cl2 (50 mL) at 0°C. After the addition was complete, the mixture was stirred at room temperature for 2 h, t ...
Oxidation of alcohols
... During the reaction, the orange potassium dichromate(Vl) solution changes to a green solution that contains chromium(lll) ions. ...
... During the reaction, the orange potassium dichromate(Vl) solution changes to a green solution that contains chromium(lll) ions. ...
Article Summaries
... The authors used model complexes in order to perform a mechanistic analysis of species closely related to those used in the Shilov system. The authors investigated reactions between [PtCl4]2- and RI (R = CH3, CH2CH2OH), which afford the corresponding [PtCl5R]2- species. Kinetic studies of the decomp ...
... The authors used model complexes in order to perform a mechanistic analysis of species closely related to those used in the Shilov system. The authors investigated reactions between [PtCl4]2- and RI (R = CH3, CH2CH2OH), which afford the corresponding [PtCl5R]2- species. Kinetic studies of the decomp ...
Baylis–Hillman reaction

The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.DABCO is one of the most frequently used tertiary amine catalysts for this reaction. In addition, nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.MBH reaction has several advantages as a useful synthetic method: 1) It is an atom-economic coupling of easily prepared starting materials. 2) Reaction of a pro-chiral electrophile generates a chiral center, therefore an asymmetric synthesis is possible. 3) Reaction products usually contain multiple functionalities in a proximity so that a variety of further transformations are possible. 4) It can employ a nucleophilic organo-catalytic system without the use of heavy metal under mild conditions.Several reviews have been written.