Alkene-Addn-PartB-2012-ques
... stereochemistry of the product, you must consider two things: (1) Stereochemistry of the starting alkene (cis or trans; Z or E) (2) Stereochemistry of the addition (syn or anti) ...
... stereochemistry of the product, you must consider two things: (1) Stereochemistry of the starting alkene (cis or trans; Z or E) (2) Stereochemistry of the addition (syn or anti) ...
Ligand to Ligand Charge Transfer in
... The emission spectrum of CuTpAsPh3 at room temperature is much lower in intensity and broader than the emission taken at 20 K, with the maximum at 20 425 cm-1 and a width at halfheight of 7280 cm-1. CuTpNEt3. The 20 K emission spectrum of CuTpNEt3 as a powder in a sealed capillary excited at 351.1 n ...
... The emission spectrum of CuTpAsPh3 at room temperature is much lower in intensity and broader than the emission taken at 20 K, with the maximum at 20 425 cm-1 and a width at halfheight of 7280 cm-1. CuTpNEt3. The 20 K emission spectrum of CuTpNEt3 as a powder in a sealed capillary excited at 351.1 n ...
Orbitals
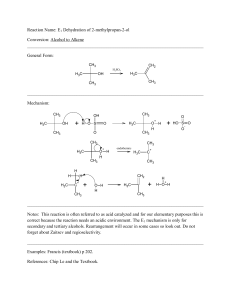
... The prefix oxo- is used if ketone is not highest priority functional group and the carbonyl is considered a substituent on the parent chain ...
... The prefix oxo- is used if ketone is not highest priority functional group and the carbonyl is considered a substituent on the parent chain ...
1 Q. What are Saturated and unsaturated hydrocarbons?imp
... One e- from s orbital is moved from 2s orbital to 2pz orbital which is in higher energy state. The input energy for exciting an electron is available from lattice energy of formation, released when bond are formed. Initially energy has to give so as to start the reaction. In the excited state one 2s ...
... One e- from s orbital is moved from 2s orbital to 2pz orbital which is in higher energy state. The input energy for exciting an electron is available from lattice energy of formation, released when bond are formed. Initially energy has to give so as to start the reaction. In the excited state one 2s ...
View/Open
... copyright hereon may be reproduced or used in any form or by any means—graphic, electronic, or mechanical, including photocopying, recording, taping, Web distribution, information storage and retrieval systems, or in any other manner—without the written permission of the publisher. Printed in the Un ...
... copyright hereon may be reproduced or used in any form or by any means—graphic, electronic, or mechanical, including photocopying, recording, taping, Web distribution, information storage and retrieval systems, or in any other manner—without the written permission of the publisher. Printed in the Un ...
Electrophilic Additions: Alkenes Addition of Hydrogen Halides
... resembles reactants (I). Endergonic reaction: late transition state resembles products (II). ...
... resembles reactants (I). Endergonic reaction: late transition state resembles products (II). ...
9: Formation of Alkenes and Alkynes. Elimination Reactions
... staggered (anti-periplanar) conformation in which the H on Cβ, and the Br on Cα , are anti to each other. Figure 9.09 You can see that when an -:OH removes the β-H, the simultaneous loss of Br:- gives the (E)-alkene as the other groups on Cα and Cβ move into the alkene plane. Similarly, the staggere ...
... staggered (anti-periplanar) conformation in which the H on Cβ, and the Br on Cα , are anti to each other. Figure 9.09 You can see that when an -:OH removes the β-H, the simultaneous loss of Br:- gives the (E)-alkene as the other groups on Cα and Cβ move into the alkene plane. Similarly, the staggere ...
Minimum Learning Competencies - Ministry of Education, Ethiopia
... velocity & the energy of an electron using Bohr model; Explain that atoms emit or absorb energy when they undergo transition from one stats to another; Explain that the spectrum of hydrogen demonstrates the quantized nature of the energy of its electron; Explain the short coming of Bohr’s theory; St ...
... velocity & the energy of an electron using Bohr model; Explain that atoms emit or absorb energy when they undergo transition from one stats to another; Explain that the spectrum of hydrogen demonstrates the quantized nature of the energy of its electron; Explain the short coming of Bohr’s theory; St ...
Ch 7 - Practice problem (Answers)
... 32. If the following E2 reaction proceeds through an anti-periplanar transition state, what product or products are expected? CH3 ...
... 32. If the following E2 reaction proceeds through an anti-periplanar transition state, what product or products are expected? CH3 ...
Chapter 22 and 23 Study Guide
... notes or help from anyone. You should review homework, quizzes and other assignments for complete test preparation. These questions are only examples. Different chemicals will be on the test. ...
... notes or help from anyone. You should review homework, quizzes and other assignments for complete test preparation. These questions are only examples. Different chemicals will be on the test. ...
Chapter 18
... 1) Ketones and aldehydes have a higher boiling point than alkanes of similar mass ...
... 1) Ketones and aldehydes have a higher boiling point than alkanes of similar mass ...
Reaction of Organometallic Reagents with Aldehydes and Ketones.
... • Since both Li and Mg are very electropositive metals, organolithium (RLi) and organomagnesium (RMgX) reagents contain very polar carbon— metal bonds and are therefore very reactive reagents. • Organomagnesium reagents are called Grignard reagents. • Organocopper reagents (R2CuLi), also called orga ...
... • Since both Li and Mg are very electropositive metals, organolithium (RLi) and organomagnesium (RMgX) reagents contain very polar carbon— metal bonds and are therefore very reactive reagents. • Organomagnesium reagents are called Grignard reagents. • Organocopper reagents (R2CuLi), also called orga ...
Oxidation of Benzyl Ethers to Benzoate Esters Using a Novel
... other aqueous solvent mixtures (Thottumkara and Vinod, 2002). Several pertinent observations were made from our initial oxidation studies. It was observed that mIBX tolerates a variety of functional groups during oxidation, and over-oxidation of alcohols is never observed, even when electron rich su ...
... other aqueous solvent mixtures (Thottumkara and Vinod, 2002). Several pertinent observations were made from our initial oxidation studies. It was observed that mIBX tolerates a variety of functional groups during oxidation, and over-oxidation of alcohols is never observed, even when electron rich su ...
Colorimetric Assay of Alditols in Complex Biological Samples
... The formation of color was completed in 1 min at 100 °C and bleaching began after 3 min at that temperature. Thus, 2 min in boiling water was chosen as the optimal condition for the color reaction. On the other hand, changing the pH of the reaction medium from 2.0 to 7.0 had only a minor effect in t ...
... The formation of color was completed in 1 min at 100 °C and bleaching began after 3 min at that temperature. Thus, 2 min in boiling water was chosen as the optimal condition for the color reaction. On the other hand, changing the pH of the reaction medium from 2.0 to 7.0 had only a minor effect in t ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.