Chapter 9
... Fluorine is too reactive to give mono-fluorinated products For Iodine, an oxidizing agent such as hydrogen peroxide or a copper salt such as CuCl2 must be added to the reaction • These substances oxidize I2 to the electrophilic species that reacts as if it were I+ • The aromatic ring reacts with the ...
... Fluorine is too reactive to give mono-fluorinated products For Iodine, an oxidizing agent such as hydrogen peroxide or a copper salt such as CuCl2 must be added to the reaction • These substances oxidize I2 to the electrophilic species that reacts as if it were I+ • The aromatic ring reacts with the ...
Chapter 8 Chemical Equations and Reactions
... Synthesis Reactions: Reactions of Elements with Oxygen One simple type of synthesis reaction is the combination of an element with oxygen to produce an oxide of the element. Almost all metals react with oxygen to form oxides. The Group 1 metals form oxides but with the formula M2O. ...
... Synthesis Reactions: Reactions of Elements with Oxygen One simple type of synthesis reaction is the combination of an element with oxygen to produce an oxide of the element. Almost all metals react with oxygen to form oxides. The Group 1 metals form oxides but with the formula M2O. ...
Efficient and catalyst-free condensation of acid chlorides and
... An efficient and catalyst-free procedure for the condensation of acyl chlorides and alcohols using continuous flow was developed. Different esters could be obtained with excellent conversions starting from the corresponding acyl chlorides and alcohols in very short reaction times (5-6.7 min). The re ...
... An efficient and catalyst-free procedure for the condensation of acyl chlorides and alcohols using continuous flow was developed. Different esters could be obtained with excellent conversions starting from the corresponding acyl chlorides and alcohols in very short reaction times (5-6.7 min). The re ...
reactions of the conjugated dienes butadiene and isoprene alone
... trimeric products evidently formed together with some alcohols and ethers suggestive of reaction between isoprene and clay interlayer water. The single most abundant species was shown to be 4-ethyl-o-xylene (C~oH14). The total conversion of isoprene was ~90% at these temperatures, but the reaction w ...
... trimeric products evidently formed together with some alcohols and ethers suggestive of reaction between isoprene and clay interlayer water. The single most abundant species was shown to be 4-ethyl-o-xylene (C~oH14). The total conversion of isoprene was ~90% at these temperatures, but the reaction w ...
Microsoft Word
... excellent yields. Substrates containing other acid labile functional groups such as acetonide, TBDMS and isopropylidene protected diols remained intact during acetylation. Interestingly, when diols were subjected to acetylation, monoacetates were formed as the major products with very good yields. F ...
... excellent yields. Substrates containing other acid labile functional groups such as acetonide, TBDMS and isopropylidene protected diols remained intact during acetylation. Interestingly, when diols were subjected to acetylation, monoacetates were formed as the major products with very good yields. F ...
AP Chem Chapter 16 Review Packet
... Answer the following questions on a separate sheet of paper. SHOW ALL WORK!! Do not assume anything - explain it! Staple your work to this paper. ...
... Answer the following questions on a separate sheet of paper. SHOW ALL WORK!! Do not assume anything - explain it! Staple your work to this paper. ...
Development of Methods for Predicting Solvation and
... molecule. This scheme systematically corrects errors in bond dipoles calculated from low-level charges, such as Löwdin population analysis charges. The CM3 charge model has several improvements over previous class IV charge models developed in our research group. In particular, the CM3 parameters we ...
... molecule. This scheme systematically corrects errors in bond dipoles calculated from low-level charges, such as Löwdin population analysis charges. The CM3 charge model has several improvements over previous class IV charge models developed in our research group. In particular, the CM3 parameters we ...
Lab 3. Chemical Reactions
... When elements or compounds chemically react to form products, no material (matter) is lost or gained. All of the atoms used as reactants are converted into products. Every atom of every element must be accounted for since they are not destroyed or created, just rearranged and recombined into new thi ...
... When elements or compounds chemically react to form products, no material (matter) is lost or gained. All of the atoms used as reactants are converted into products. Every atom of every element must be accounted for since they are not destroyed or created, just rearranged and recombined into new thi ...
11.2 Types of Chemical Reactions
... The five general types of reaction are combination, decomposition, single-replacement, double-replacement, and combustion. Not all chemical reactions fit uniquely into only one category. Occasionally, a reaction may fit equally well into two categories. Nevertheless, recognizing a reaction as a parti ...
... The five general types of reaction are combination, decomposition, single-replacement, double-replacement, and combustion. Not all chemical reactions fit uniquely into only one category. Occasionally, a reaction may fit equally well into two categories. Nevertheless, recognizing a reaction as a parti ...
Chapter 19 - people.vcu.edu
... If the amine is not the high-priority group, then the nitrogen is named as an amino substituent. ...
... If the amine is not the high-priority group, then the nitrogen is named as an amino substituent. ...
kinetics and equilibrium
... Rate = k [NO]2 [Cl2] What is the order of the reaction with respect to NO? • 2nd order • What is the order of the reaction with respect to Cl2 • 1st order ...
... Rate = k [NO]2 [Cl2] What is the order of the reaction with respect to NO? • 2nd order • What is the order of the reaction with respect to Cl2 • 1st order ...
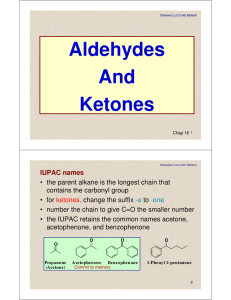
Aldehydes And Ketones
... a carbonyl is a new chiral center – if none of the starting materials is chiral and the reaction takes place in an achiral environment, then enantiomers will be formed as a racemic mixture Ap proach from the top face ...
... a carbonyl is a new chiral center – if none of the starting materials is chiral and the reaction takes place in an achiral environment, then enantiomers will be formed as a racemic mixture Ap proach from the top face ...
Document
... Polar compds. Higher bp than non-polar compds of similar M r. Lower bp than acid and alc. because no H bonding ...
... Polar compds. Higher bp than non-polar compds of similar M r. Lower bp than acid and alc. because no H bonding ...
Experiment 15: Reduction and Oxidation of Organic Compounds
... mouth of the flask, and attach the side-arm to the vacuum outlet. Slowly open the valve. Using a steam bath, evaporate the ether under vacuum. Turn off the vacuum as soon as all the liquid has evaporated (or the product will sublime). Weigh the flask with the crude product. You will purify the camph ...
... mouth of the flask, and attach the side-arm to the vacuum outlet. Slowly open the valve. Using a steam bath, evaporate the ether under vacuum. Turn off the vacuum as soon as all the liquid has evaporated (or the product will sublime). Weigh the flask with the crude product. You will purify the camph ...
Equilibrium (Sheet 1)
... CO, H2, and CO2 will not change. Now then, assume the concentration of H2O was increased, then effectively the number of collisions between H2O molecules and CO molecules are increased, resulting in an increase in the number of molecules possessing the activation energy, thus the rate of the forward ...
... CO, H2, and CO2 will not change. Now then, assume the concentration of H2O was increased, then effectively the number of collisions between H2O molecules and CO molecules are increased, resulting in an increase in the number of molecules possessing the activation energy, thus the rate of the forward ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.