chapter 5 - chemical reactions
... 3. Indicate the state of substances: (g) for gas, (l) for liquid, (s) for solid, and (aq) for aqueous solution. 4. Balance the equation by introducing smallest integer (whole number) coefficients in front of each reactant and product as needed, (coefficient "1" is not shown). The chemical formula of ...
... 3. Indicate the state of substances: (g) for gas, (l) for liquid, (s) for solid, and (aq) for aqueous solution. 4. Balance the equation by introducing smallest integer (whole number) coefficients in front of each reactant and product as needed, (coefficient "1" is not shown). The chemical formula of ...
Chem 401 Lab Exercise #5 Nomenclature Worksheet for Alkanes
... geometric isomers). This type of stereoisomer occurs when there is a source of rigidity in the molecule. Cycloalkanes and alkenes both exhibit cis-trans isomers. In cycloalkanes, the closed ring structure restricts rotation about the C-C bond, so the 2 substituents on each ring C may point up or dow ...
... geometric isomers). This type of stereoisomer occurs when there is a source of rigidity in the molecule. Cycloalkanes and alkenes both exhibit cis-trans isomers. In cycloalkanes, the closed ring structure restricts rotation about the C-C bond, so the 2 substituents on each ring C may point up or dow ...
i principi di base - Structural Biology
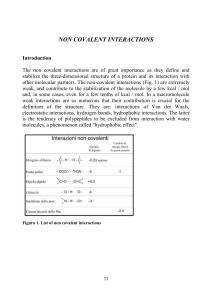
... Table IV shows the association constant for a number of small molecules, i.e. their capacity to associate one with the other when placed in an aqueous solvent. The association constant (which is measured in M-1) is relatively high for molecules that are able to make salt bridges and is of the same o ...
... Table IV shows the association constant for a number of small molecules, i.e. their capacity to associate one with the other when placed in an aqueous solvent. The association constant (which is measured in M-1) is relatively high for molecules that are able to make salt bridges and is of the same o ...
SYNOPSIS
... Chapter III: Lithium perchlorate catalyzed reactions of indoles: An expeditious synthesis of Bis (indolyl) methanes. The acid-catalyzed reaction of electron-rich heterocyclic compounds with pdimethylamino benzaldehyde is known as Ehrlich test for -electron excessive heterocycles such as pyroles and ...
... Chapter III: Lithium perchlorate catalyzed reactions of indoles: An expeditious synthesis of Bis (indolyl) methanes. The acid-catalyzed reaction of electron-rich heterocyclic compounds with pdimethylamino benzaldehyde is known as Ehrlich test for -electron excessive heterocycles such as pyroles and ...
Alcohols Oxidation by oxygen O2 in presence of
... more active to oxidation and product were generated in shorter time with high yield. On the other hand, the benzylic alcohols having acceptor groups such as on the phenyl ring have had low activities. Also the benzylic alcohols type 2 such as1phenyl alcohols and benzhydrol has been oxidized slower t ...
... more active to oxidation and product were generated in shorter time with high yield. On the other hand, the benzylic alcohols having acceptor groups such as on the phenyl ring have had low activities. Also the benzylic alcohols type 2 such as1phenyl alcohols and benzhydrol has been oxidized slower t ...
Document
... The hydrogens are enantiotopic and equivalent in the NMR unless the molecule is placed in a chiral environment such as a ...
... The hydrogens are enantiotopic and equivalent in the NMR unless the molecule is placed in a chiral environment such as a ...
Ch04-04-alkenes-2
... Exergonic reaction: early transition state resembles reactants (I). Endergonic reaction: late transition state resembles products (II). ...
... Exergonic reaction: early transition state resembles reactants (I). Endergonic reaction: late transition state resembles products (II). ...
File
... Therefore enzymes are catalysts because they speed up biochemical reactions • We need enzymes for every process that happens in our bodies! e.g. Digesting food, replicating DNA ...
... Therefore enzymes are catalysts because they speed up biochemical reactions • We need enzymes for every process that happens in our bodies! e.g. Digesting food, replicating DNA ...
Multiple Pathways To Success Quarter 3 Learning Module
... Students will construct covalent bonds using molecular bonding theory. Students will explore bonding theory and use this information to build molecular compounds. Students will analyze polar and nonpolar compounds and their interactions. Students will apply polar / nonpolar concepts to solve polar m ...
... Students will construct covalent bonds using molecular bonding theory. Students will explore bonding theory and use this information to build molecular compounds. Students will analyze polar and nonpolar compounds and their interactions. Students will apply polar / nonpolar concepts to solve polar m ...
Alkenes 3 - ChemWeb (UCC)
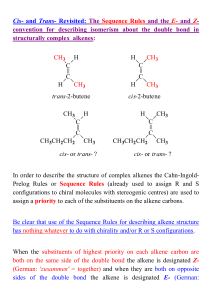
... In order to describe the structure of complex alkenes the Cahn-IngoldPrelog Rules or Sequence Rules (already used to assign R and S configurations to chiral molecules with stereogenic centres) are used to assign a priority to each of the substituents on the alkene carbons. Be clear that use of the S ...
... In order to describe the structure of complex alkenes the Cahn-IngoldPrelog Rules or Sequence Rules (already used to assign R and S configurations to chiral molecules with stereogenic centres) are used to assign a priority to each of the substituents on the alkene carbons. Be clear that use of the S ...
Stoichiometry intro
... Using the Mole Ratio Remember that the coefficients from a balanced reaction represent the ratio of the moles of substances that react and form during a chemical reaction. These numbers are fixed - they do not change We can use these ratios to predict the amounts of substances that react and fo ...
... Using the Mole Ratio Remember that the coefficients from a balanced reaction represent the ratio of the moles of substances that react and form during a chemical reaction. These numbers are fixed - they do not change We can use these ratios to predict the amounts of substances that react and fo ...
File - Mr. Heff`s Class
... - Occurs when two or more equal valid structures can be drawn for the molecule. - The single and double bonds appear to oscillate between two sets of positions. ...
... - Occurs when two or more equal valid structures can be drawn for the molecule. - The single and double bonds appear to oscillate between two sets of positions. ...
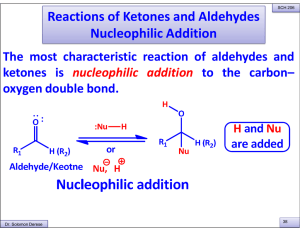
Reactions of Ketones and Aldehydes Nucleophilic Addition
... If the cassava is crushed with water and allowed to stand (‘ferment’), enzymes in the cassava will do the same job and then the HCN can be washed out before the cassava is cooked and eaten. The cassava is now safe to eat but it still contains some glucoside. Some diseases found in eastern Nigeria ca ...
... If the cassava is crushed with water and allowed to stand (‘ferment’), enzymes in the cassava will do the same job and then the HCN can be washed out before the cassava is cooked and eaten. The cassava is now safe to eat but it still contains some glucoside. Some diseases found in eastern Nigeria ca ...
PDF document
... above all in carbon-carbon bond formation, mainly through metal catalysed coupling reactions. In addition, many iodinated aromatic derivatives are used in medicine as drugs or diagnostic aids, contrasting agents, and radioactively labelled markers. The chemistry dealing with selective iodination of ...
... above all in carbon-carbon bond formation, mainly through metal catalysed coupling reactions. In addition, many iodinated aromatic derivatives are used in medicine as drugs or diagnostic aids, contrasting agents, and radioactively labelled markers. The chemistry dealing with selective iodination of ...
I - Holland Public Schools
... * What does collision theory say? In order for a reaction to occur, the reactant particles must physically collide with each other In this case, 2 C2H2’s and 5 O2’s would need to collide in the same place at the same time VERY UNLIKELY * OK, so how does this work then? The chemical reaction is divid ...
... * What does collision theory say? In order for a reaction to occur, the reactant particles must physically collide with each other In this case, 2 C2H2’s and 5 O2’s would need to collide in the same place at the same time VERY UNLIKELY * OK, so how does this work then? The chemical reaction is divid ...
Organic Chemistry
... alkenes react with H2 in the presence of a transition metal catalyst to give alkanes + H2 Cyclohexene ...
... alkenes react with H2 in the presence of a transition metal catalyst to give alkanes + H2 Cyclohexene ...
Hydrogenation of Amino Acid Mixtures to Amino Alcohols
... enhancing their utility The development of such routes to use renewables is desirable in light of the strain on petroleum derived feedstocks. Catalytic hydrogenation of individual amino acids has been studied previously in our laboratory1-4 and elsewhere5,6. Given the complex composition of most bio ...
... enhancing their utility The development of such routes to use renewables is desirable in light of the strain on petroleum derived feedstocks. Catalytic hydrogenation of individual amino acids has been studied previously in our laboratory1-4 and elsewhere5,6. Given the complex composition of most bio ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.