practice unit #2 exam
... 50 on the exam than is will be expected provided on this review - make sure you go through the 820 page assignment on Reaction Mechanisms ...
... 50 on the exam than is will be expected provided on this review - make sure you go through the 820 page assignment on Reaction Mechanisms ...
6.02 × 1023 molecules = 1 mole
... convert mass percent of each element to a molar ratio of each element. Step 1 Assume your unknown sample has a mass of 100 grams. ...
... convert mass percent of each element to a molar ratio of each element. Step 1 Assume your unknown sample has a mass of 100 grams. ...
Chapter 3 Chemical Reactions
... achieved, the amount of each reactant and product remains constant. ...
... achieved, the amount of each reactant and product remains constant. ...
chemical reaction
... • How to Balance an Equation To balance an equation, you must use coefficients. A coefficient is a number that is placed in front of a chemical symbol or formula. • For an equation to be balanced, all atoms must be counted. So, you multiply the subscript of each element in a formula by the formula’s ...
... • How to Balance an Equation To balance an equation, you must use coefficients. A coefficient is a number that is placed in front of a chemical symbol or formula. • For an equation to be balanced, all atoms must be counted. So, you multiply the subscript of each element in a formula by the formula’s ...
Monomer
... ⇒ -TiCl3 (brown color) was found to be effective for the polymerization (prepared by mixing TiCl4 with H2, aluminium, or alkylaluminiums Still isotacticity 20 – 40 % with Al(C2H5)2Cl -, -, -crystals of TiCl3 were found to increase the isoctacticity ...
... ⇒ -TiCl3 (brown color) was found to be effective for the polymerization (prepared by mixing TiCl4 with H2, aluminium, or alkylaluminiums Still isotacticity 20 – 40 % with Al(C2H5)2Cl -, -, -crystals of TiCl3 were found to increase the isoctacticity ...
Aromatic electrophilic substitution
... a combined effect is observed in subsequent reactions. 2. In many cases it is easy to predict the effects of multiple substituent groups because the individual effects are mutually supporting of each other. 3. In cases were there is a conflict in the directing effects of the substituent groups it ca ...
... a combined effect is observed in subsequent reactions. 2. In many cases it is easy to predict the effects of multiple substituent groups because the individual effects are mutually supporting of each other. 3. In cases were there is a conflict in the directing effects of the substituent groups it ca ...
Chapter 5
... species, coordination of the acetylene moiety, creation of the new C allyl -Cacetylene bond, carbonyl insertion, and ring closing to form either five- or six-member rings, mainly cyclopentenones and cyclohexenones. In this study, we have explored the different steps that presumably constitute the ov ...
... species, coordination of the acetylene moiety, creation of the new C allyl -Cacetylene bond, carbonyl insertion, and ring closing to form either five- or six-member rings, mainly cyclopentenones and cyclohexenones. In this study, we have explored the different steps that presumably constitute the ov ...
16 Alcohols, Phenols, Aldehydes
... Primary alcohols (1o) easily oxidize to form aldehydes with can then oxidize to form carboxylic acids. Secondary alcohols (2o) oxidize to ketones. Tertiary alcohols (3o) do not oxidize. Oxidation of alcohols or aldehydes requires an oxidizing agent that itself will get reduced. A common oxidizing ag ...
... Primary alcohols (1o) easily oxidize to form aldehydes with can then oxidize to form carboxylic acids. Secondary alcohols (2o) oxidize to ketones. Tertiary alcohols (3o) do not oxidize. Oxidation of alcohols or aldehydes requires an oxidizing agent that itself will get reduced. A common oxidizing ag ...
Problems - Department of Chemistry HKU
... where p0 is the initial pressure and p is the final pressure of cyclopropane. What is the order and rate constant for the reaction under these conditions? 21.10 The addition of hydrogen halides to alkenes has played a fundamental role in the investigation of organic reaction mechanisms. In one study ...
... where p0 is the initial pressure and p is the final pressure of cyclopropane. What is the order and rate constant for the reaction under these conditions? 21.10 The addition of hydrogen halides to alkenes has played a fundamental role in the investigation of organic reaction mechanisms. In one study ...
File - mrs. whalen`s classes!
... a. Primary: -OH bonded to a C that is bonded to only one other C b. Secondary: -OH bonded to a C that is bonded to 2 other C’s c. Tertiary: -OH bonded to a C that is bonded to 3 other C’s Naming: 1. Find the longest chain containing the C the –OH is bonded to. 2. Replace the “e” at the end of the pa ...
... a. Primary: -OH bonded to a C that is bonded to only one other C b. Secondary: -OH bonded to a C that is bonded to 2 other C’s c. Tertiary: -OH bonded to a C that is bonded to 3 other C’s Naming: 1. Find the longest chain containing the C the –OH is bonded to. 2. Replace the “e” at the end of the pa ...
Novel Strecker Degradation Products of Tyrosine and
... diseases has been established for a long time (DEGENHARDT et al. 1998). Tyrosine (Tyr) accompanies phenylalanine in the majority of proteins and its average content is about 3.5%. Enzymatic oxidation of Tyr in some food products such as beans and mushrooms, in the so called nonenzymatic browning rea ...
... diseases has been established for a long time (DEGENHARDT et al. 1998). Tyrosine (Tyr) accompanies phenylalanine in the majority of proteins and its average content is about 3.5%. Enzymatic oxidation of Tyr in some food products such as beans and mushrooms, in the so called nonenzymatic browning rea ...
II. Chemistry of Sugars
... one of these C atoms has a substituent with a higher priority than the other. If this is the case, the C atom bearing the higher priority substituent is assigned to b; the other is then c, e.g., for the two underlined C atoms, -C H2CH3 has a higher priority than - C H3 because in the former one of t ...
... one of these C atoms has a substituent with a higher priority than the other. If this is the case, the C atom bearing the higher priority substituent is assigned to b; the other is then c, e.g., for the two underlined C atoms, -C H2CH3 has a higher priority than - C H3 because in the former one of t ...
Hydrogenation, Transfer Hydrogenat- ion and Hydrogen Transfer Reactions
... Catalysis is the process that facilitates a chemical reaction, and was first introduced by Jöns Jacob Berzelius in 1835.2 A catalyst is an additive that triggers and participates in the reaction to make the reaction go faster by decreasing the activation energy, without itself being consumed. It doe ...
... Catalysis is the process that facilitates a chemical reaction, and was first introduced by Jöns Jacob Berzelius in 1835.2 A catalyst is an additive that triggers and participates in the reaction to make the reaction go faster by decreasing the activation energy, without itself being consumed. It doe ...
Functional Group Isomerism
... In fuel design, the most useful octane isomers are those which have "branched" structures. These isomers are distinguished from those that have more linear structures. A linear isomer has all its elements connected in a line, whereas a branched may resemble a tree in structure, with each element not ...
... In fuel design, the most useful octane isomers are those which have "branched" structures. These isomers are distinguished from those that have more linear structures. A linear isomer has all its elements connected in a line, whereas a branched may resemble a tree in structure, with each element not ...
Organic Chemistry
... Crude Oil: Complex mixture of 100’s of hydrocarbon variants →“Cracking”: Separate mixture out by boiling point ...
... Crude Oil: Complex mixture of 100’s of hydrocarbon variants →“Cracking”: Separate mixture out by boiling point ...
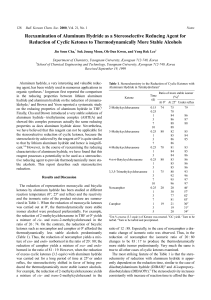
Reexamination of Aluminum Hydride as a Stereoselective Reducing
... ketones by aluminum hydride has been studied at different reaction temperature (0o, 25o and reflux) and the reactivity and the isomeric ratio of the product mixture are summarized in Table 1. When the reduction of monocyclic ketones was carried out at 0o, the thermodynamically more stable isomer alc ...
... ketones by aluminum hydride has been studied at different reaction temperature (0o, 25o and reflux) and the reactivity and the isomeric ratio of the product mixture are summarized in Table 1. When the reduction of monocyclic ketones was carried out at 0o, the thermodynamically more stable isomer alc ...
Chapter 9
... Fluorine is too reactive to give mono-fluorinated products For Iodine, an oxidizing agent such as hydrogen peroxide or a copper salt such as CuCl2 must be added to the reaction • These substances oxidize I2 to the electrophilic species that reacts as if it were I+ • The aromatic ring reacts with the ...
... Fluorine is too reactive to give mono-fluorinated products For Iodine, an oxidizing agent such as hydrogen peroxide or a copper salt such as CuCl2 must be added to the reaction • These substances oxidize I2 to the electrophilic species that reacts as if it were I+ • The aromatic ring reacts with the ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.