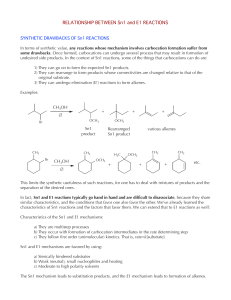
RELATIONSHIP BETWEEN Sn1 and E1 REACTIONS
... Why is the trisubstituted alkene more stable than the other two? The relative stabilities of alkenes is measured by their heat of hydrogenation (much like the relative stabilities of cycloalkanes is measured by their heats of combustion, see ch. 3). The relationship is inverse: the higher the heat o ...
... Why is the trisubstituted alkene more stable than the other two? The relative stabilities of alkenes is measured by their heat of hydrogenation (much like the relative stabilities of cycloalkanes is measured by their heats of combustion, see ch. 3). The relationship is inverse: the higher the heat o ...
Le Chatelier`s Principle Notes
... If more Fe3+ is added to the reaction, what will happen? According to Le Châtelier's Principle, the system will react to minimize the stress. Since Fe3+ is on the reactant side of this reaction, the rate of the forward reaction will increase in order to "use up" the additional reactant. This will ca ...
... If more Fe3+ is added to the reaction, what will happen? According to Le Châtelier's Principle, the system will react to minimize the stress. Since Fe3+ is on the reactant side of this reaction, the rate of the forward reaction will increase in order to "use up" the additional reactant. This will ca ...
Module 3 -- Lesson 4
... yield. The conditions which produce the highest yield are called the optimum conditions. For example, the optimum conditions for the Haber process are: ...
... yield. The conditions which produce the highest yield are called the optimum conditions. For example, the optimum conditions for the Haber process are: ...
Phenomenological description of the transition state, and the bond
... chemical reaction based on the valence-bond theory (VB) through the avoided crossing state (ACS) or the perfectly resonating state (PRS) of the VB configurations that describe a chemical transformation [20, 21]. The PRS is typified by a pairwise resonance (or avoided crossing) between two of the pri ...
... chemical reaction based on the valence-bond theory (VB) through the avoided crossing state (ACS) or the perfectly resonating state (PRS) of the VB configurations that describe a chemical transformation [20, 21]. The PRS is typified by a pairwise resonance (or avoided crossing) between two of the pri ...
C–H Bond Functionalization in Complex Organic Synthesis REVIEW
... in many different structural contexts by the proper choice of a chiral dirhodium catalyst (Fig. 6A) (11). These reactions are frequently applied to the synthesis of advanced intermediates and natural products (12). Several features make them attractive in this respect, including neutral reaction con ...
... in many different structural contexts by the proper choice of a chiral dirhodium catalyst (Fig. 6A) (11). These reactions are frequently applied to the synthesis of advanced intermediates and natural products (12). Several features make them attractive in this respect, including neutral reaction con ...
Fundamentals Of Organic Chemistry
... Reactions involving the change in the carbon skeleton through the rearrangement of the carbonium intermediate by alkyl and hydride shift collectively known as Wagner–Meerwein rearrangement. When neopentyl bromide is hydrolysed under S N1 (due to bulky alkyl group) condition it is found that instead ...
... Reactions involving the change in the carbon skeleton through the rearrangement of the carbonium intermediate by alkyl and hydride shift collectively known as Wagner–Meerwein rearrangement. When neopentyl bromide is hydrolysed under S N1 (due to bulky alkyl group) condition it is found that instead ...
No Slide Title - McMaster Chemistry
... In Br nsted acid-base reactions - H+ transfer occurs from STRONGER to WEAKER congugate acid-base pair 1A03/1E03 Types of Reactions (2) ...
... In Br nsted acid-base reactions - H+ transfer occurs from STRONGER to WEAKER congugate acid-base pair 1A03/1E03 Types of Reactions (2) ...
Test 9 Review - Evan`s Chemistry Corner
... Entropy. Entropy is randomness or disorder. In nature, high entropy is favored, yet many things that occur naturally are quite organized. This is because nature also favors low potential energy or enthalpy. Entropy and enthalpy sometimes conflict with each other. In order to determine if a reaction ...
... Entropy. Entropy is randomness or disorder. In nature, high entropy is favored, yet many things that occur naturally are quite organized. This is because nature also favors low potential energy or enthalpy. Entropy and enthalpy sometimes conflict with each other. In order to determine if a reaction ...
Catalytic, Enantioselective Alkylations of N,O- and
... Mechanism. To our surprise, the use of one equiv enol silane 4a with N,O-acetal l a did not lead to product 5a with 5 mol% 2; however, when two equiv were used, product 5a was formed in good yield. Although silyl ketene acetals can be quenched through silyl transfer reactions with alcohols, enol sil ...
... Mechanism. To our surprise, the use of one equiv enol silane 4a with N,O-acetal l a did not lead to product 5a with 5 mol% 2; however, when two equiv were used, product 5a was formed in good yield. Although silyl ketene acetals can be quenched through silyl transfer reactions with alcohols, enol sil ...
SECONDARY METABOLISM: THE BUILDING BLOCKS AND
... of a natural product. As we shall see, oxygen atoms can be introduced and removed by a variety of processes, and so are not considered in the initial analysis, except as a pointer to an acetate (see page 62) or shikimate (see page 123) origin. The structural features of these building blocks are sho ...
... of a natural product. As we shall see, oxygen atoms can be introduced and removed by a variety of processes, and so are not considered in the initial analysis, except as a pointer to an acetate (see page 62) or shikimate (see page 123) origin. The structural features of these building blocks are sho ...
Chapter 14
... At first glance this looks impossible. How are we to break one of the unactivated C-C single bonds in cyclohexane to make a 1,6-diol with two extra carbons! So, rather than looking at the starting material, look at the target molecule and look at the first disconnection shown by the wavy line. If we ...
... At first glance this looks impossible. How are we to break one of the unactivated C-C single bonds in cyclohexane to make a 1,6-diol with two extra carbons! So, rather than looking at the starting material, look at the target molecule and look at the first disconnection shown by the wavy line. If we ...
An Epoxidation Reaction: The Epoxidation of Cholesterol to 5 ,6
... In this experiment, we will prepare an epoxide or oxirane by epoxidizing chlolesterol. Cholesterol is the most common compound in the class of compounds called sterols. As the name implies, sterols contain a hydroxyl (-OH) group. Because sterols are alcohols, they have the characteristic name ending ...
... In this experiment, we will prepare an epoxide or oxirane by epoxidizing chlolesterol. Cholesterol is the most common compound in the class of compounds called sterols. As the name implies, sterols contain a hydroxyl (-OH) group. Because sterols are alcohols, they have the characteristic name ending ...
ConcepTest On Simple Redox Reactions
... Comment to Instructor: Correct answer is 3. HCl. Since the oxidation number of H is decreasing from +1 to 0, it is undergoing reduction. Zn is being oxidized, and HCl is the “agent” that is causing the Zn to be oxidized. #4 indicates that the student is thinking that the Zn+2in ZnCl2 is undergoing r ...
... Comment to Instructor: Correct answer is 3. HCl. Since the oxidation number of H is decreasing from +1 to 0, it is undergoing reduction. Zn is being oxidized, and HCl is the “agent” that is causing the Zn to be oxidized. #4 indicates that the student is thinking that the Zn+2in ZnCl2 is undergoing r ...
Enzymatic synthesis of sialic acid derivative by immobilized lipase
... RM IM, the yield after 6 h was 62.7% lower than that after synthesis catalyzed by Novozym 435. Higher activity of Novozym 435 was also found in the kinetic resolution of secondary alcohols in monoether-functionalized ionic liquids (Zhou et al., 2011). Thus, Novozym 435 was observed to efficiently cat ...
... RM IM, the yield after 6 h was 62.7% lower than that after synthesis catalyzed by Novozym 435. Higher activity of Novozym 435 was also found in the kinetic resolution of secondary alcohols in monoether-functionalized ionic liquids (Zhou et al., 2011). Thus, Novozym 435 was observed to efficiently cat ...
4888 Journal of the American Chemical Society 1OO:lS 1 July 19
... for the matrix-isolated species, more recent calculations conclude that the ST lies above the SS in the energy surface of the system and a rectangular singlet (RS) represents the ground state of l . 8 3 9 Earlier we emphasized the necessity of further IR studies on both 1 and its perdeuterio derivat ...
... for the matrix-isolated species, more recent calculations conclude that the ST lies above the SS in the energy surface of the system and a rectangular singlet (RS) represents the ground state of l . 8 3 9 Earlier we emphasized the necessity of further IR studies on both 1 and its perdeuterio derivat ...
Document
... Pre-steady-state kinetics vs steady-state kinetics 1. The order of binding of substrates and release of product serves to define the reactants present at the active site during catalysis: it does not establish the kinetically preferred order of substrate addition and product release or allow conclu ...
... Pre-steady-state kinetics vs steady-state kinetics 1. The order of binding of substrates and release of product serves to define the reactants present at the active site during catalysis: it does not establish the kinetically preferred order of substrate addition and product release or allow conclu ...
Chapter 4 Quantities of Reactants and Products 4.1 Chemical
... Combustion Analysis Problem #1 Benzene is a liquid compound composed of carbon and hydrogen. A sample of benzene weighing 342 mg is burned in excess oxygen and forms 1156 mg of carbon dioxide. What is the percent composition of benzene? [92.25%C & 7.75% H] Combustion Analysis Problem #2 n-Butyl phth ...
... Combustion Analysis Problem #1 Benzene is a liquid compound composed of carbon and hydrogen. A sample of benzene weighing 342 mg is burned in excess oxygen and forms 1156 mg of carbon dioxide. What is the percent composition of benzene? [92.25%C & 7.75% H] Combustion Analysis Problem #2 n-Butyl phth ...
7. Alkenes: Reactions and Synthesis
... • Reduction in general is addition of H2 or its equivalent • Requires Pt or Pd as powders on carbon and H2 • Hydrogen is first adsorbed on catalyst • Reaction is heterogeneous (process is not in solution) ...
... • Reduction in general is addition of H2 or its equivalent • Requires Pt or Pd as powders on carbon and H2 • Hydrogen is first adsorbed on catalyst • Reaction is heterogeneous (process is not in solution) ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.























