Aldehydes and Ketones
... • Carbonyl compound: Any compound that contains a carbonyl group, C=O. • Carbonyl group: A functional group that has a C atom joined to an O atom by a double bond. • The bond angles between the three substituents on the carbonyl carbon atom are 120°, or close to it. ...
... • Carbonyl compound: Any compound that contains a carbonyl group, C=O. • Carbonyl group: A functional group that has a C atom joined to an O atom by a double bond. • The bond angles between the three substituents on the carbonyl carbon atom are 120°, or close to it. ...
Highly Enantioselective Cyclocarbonylation of Allylic
... We have carried out cyclocarbonylation of various aliphatic and aromatic unsaturated allylic alcohols catalyzed by Pd-BICP and Pd-Xyl-BICP complexes under the optimal reaction conditions (Table 1). In general, enantioselectivity in the cyclocarbonylation of several of the allylic alcohols with the P ...
... We have carried out cyclocarbonylation of various aliphatic and aromatic unsaturated allylic alcohols catalyzed by Pd-BICP and Pd-Xyl-BICP complexes under the optimal reaction conditions (Table 1). In general, enantioselectivity in the cyclocarbonylation of several of the allylic alcohols with the P ...
Naming the Alkenes
... Rule 5: Use the IUPAC E,Z system when cis/trans labels are not applicable (3 or 4 different substituents attached to the double-bond carbons). Apply the sequence rules devised for R,S substituent priorities to the two groups on each double-bond carbon. If the two groups of highest priority are on o ...
... Rule 5: Use the IUPAC E,Z system when cis/trans labels are not applicable (3 or 4 different substituents attached to the double-bond carbons). Apply the sequence rules devised for R,S substituent priorities to the two groups on each double-bond carbon. If the two groups of highest priority are on o ...
Classification of Halogen Derivatives
... 4. Haloalkanes though polar but are insoluble in water as they do not form hydrogen bonding with water. 5. Density order is RI > RBr > RCl > RF (For the same alkyl group) CH3I > C2H5I > C3H7I Chemical Reactions of Haloalkanes 1. Nucleophilic Substitution Reactions (SN reactions) ...
... 4. Haloalkanes though polar but are insoluble in water as they do not form hydrogen bonding with water. 5. Density order is RI > RBr > RCl > RF (For the same alkyl group) CH3I > C2H5I > C3H7I Chemical Reactions of Haloalkanes 1. Nucleophilic Substitution Reactions (SN reactions) ...
Alcohol Worksheet Key
... groups at position 3 and 5 will have very little effect on the acidity of the phenol. ...
... groups at position 3 and 5 will have very little effect on the acidity of the phenol. ...
Chemical Reactions
... • The only place you can change any number is the coefficient. • A coefficient is a number written in front of a chemical formula. • Don’t forget diatomic molecules. • Use the smallest ratio of coefficients possible. ...
... • The only place you can change any number is the coefficient. • A coefficient is a number written in front of a chemical formula. • Don’t forget diatomic molecules. • Use the smallest ratio of coefficients possible. ...
Aldehydes & Ketones
... • The aldehydes and ketones are characterized by the presence of the carbonyl group. • The carbonyl group is a functional group made up of a carbon atom bonded to an oxygen atom by a double bond. • Carbonyl compounds are those that contain a carbonyl group. • Aldehydes, ketones, carboxylic acids, an ...
... • The aldehydes and ketones are characterized by the presence of the carbonyl group. • The carbonyl group is a functional group made up of a carbon atom bonded to an oxygen atom by a double bond. • Carbonyl compounds are those that contain a carbonyl group. • Aldehydes, ketones, carboxylic acids, an ...
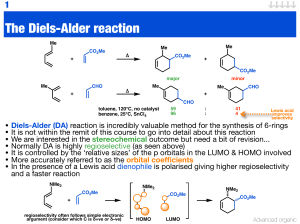
The Diels-Alder reaction
... • Diels-Alder (DA) reaction is incredibly valuable method for the synthesis of 6-rings • It is not within the remit of this course to go into detail about this reaction • We are interested in the stereochemical outcome but need a bit of revision... • Normally DA is highly regioselective (as seen abo ...
... • Diels-Alder (DA) reaction is incredibly valuable method for the synthesis of 6-rings • It is not within the remit of this course to go into detail about this reaction • We are interested in the stereochemical outcome but need a bit of revision... • Normally DA is highly regioselective (as seen abo ...
Organic Synthesis Part 2
... As we've already seen, addition of hydride to unsymmetrical ketones gives rise to an asymmetric centre. Reaction of achiral reagents with ketones containing no existing asymmetric centres gives rise to a racemic mixture. This can be altered by the use of chiral reagents (or catalysts) - see later. I ...
... As we've already seen, addition of hydride to unsymmetrical ketones gives rise to an asymmetric centre. Reaction of achiral reagents with ketones containing no existing asymmetric centres gives rise to a racemic mixture. This can be altered by the use of chiral reagents (or catalysts) - see later. I ...
Organic Chemistry 1 1st Hour Exam Student ID # Name
... (b) Explain why one product is the major isomer based on their reaction coordinate diagrams that show the two different reaction progresses (or pathways) to give the two different products, the major and the minor products. Explain the reaction results using the Hammond postulate. ...
... (b) Explain why one product is the major isomer based on their reaction coordinate diagrams that show the two different reaction progresses (or pathways) to give the two different products, the major and the minor products. Explain the reaction results using the Hammond postulate. ...
2009_outline_4
... 1. From Acid Halides and Carboxylates 2. Cyclic Dehydration of Diacids C. Reactions on Carbonyl Carbon 1. Hydrolysis to Carboxylic Acids 2. Alcoholysis to Ester and Acid 3. Ammonolysis to Amide and Salt of Acid 4. Reduction to Alcohols ...
... 1. From Acid Halides and Carboxylates 2. Cyclic Dehydration of Diacids C. Reactions on Carbonyl Carbon 1. Hydrolysis to Carboxylic Acids 2. Alcoholysis to Ester and Acid 3. Ammonolysis to Amide and Salt of Acid 4. Reduction to Alcohols ...
Aldehydes and Ketones
... • The sp2 hybridized C–H proton of an aldehyde is highly deshielded and absorbs far downfield at 9–10 ppm. • Splitting occurs with protons on the α carbon, but the coupling constant is often very small (J = 1–3 Hz). • Protons on the α carbon to the carbonyl group absorb at ...
... • The sp2 hybridized C–H proton of an aldehyde is highly deshielded and absorbs far downfield at 9–10 ppm. • Splitting occurs with protons on the α carbon, but the coupling constant is often very small (J = 1–3 Hz). • Protons on the α carbon to the carbonyl group absorb at ...
a. Rank by acidity. The most acidic compound is 1, wh
... Why does one glycol not react with periodic acid? (6 points) In the second reaction a trans stereochemistry is obtained for the glycol. The periodate cannot form with a trans stereochemistry of the two alcohols. 3.(8) Consider the reaction shown. Indicate a mechanism, by showing intermediates obtain ...
... Why does one glycol not react with periodic acid? (6 points) In the second reaction a trans stereochemistry is obtained for the glycol. The periodate cannot form with a trans stereochemistry of the two alcohols. 3.(8) Consider the reaction shown. Indicate a mechanism, by showing intermediates obtain ...
Name: Chem 22 Final exam Spring `00 What product is formed when
... 3. Which of the following statements is true? a) Thiols have a pka values of about 16, and they are stronger acids than alcohols. b) Thiols have a pka values of about 16, and they are weaker acids than alcohols c) Thiols have a pka values of about 10, and they are stronger acids than alcohols d) Thi ...
... 3. Which of the following statements is true? a) Thiols have a pka values of about 16, and they are stronger acids than alcohols. b) Thiols have a pka values of about 16, and they are weaker acids than alcohols c) Thiols have a pka values of about 10, and they are stronger acids than alcohols d) Thi ...
ALDEHYDES & KETONES - Rogue Community College
... NOT as part of ... Aliphatic rings or Aromatic rings ...
... NOT as part of ... Aliphatic rings or Aromatic rings ...
haloalkanes (halogenoalkanes)
... process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 is favoured for tertiary haloalkanes where there is steric hindrance to the attack and a more ...
... process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 is favoured for tertiary haloalkanes where there is steric hindrance to the attack and a more ...
U. of Kentucky Chemistry 535 Synthetic Organic Chemistry Spring
... retrosynthetic analysis that leaves no doubt for the reader that you can make the molecule. You may start with molecules containing no less than eight carbon atoms. ...
... retrosynthetic analysis that leaves no doubt for the reader that you can make the molecule. You may start with molecules containing no less than eight carbon atoms. ...
Review
... Mono-substituted cyclohexanes: equatorial substituents are more stable (1,3-diaxial interactions); tert-butylcyclohexane Disubstituted cyclohexanes: cis/trans-isomerism; comparison of stability (the fewer are the axial substituents, the more stable is the disubstituted cyclohexane. Fused rings (cis ...
... Mono-substituted cyclohexanes: equatorial substituents are more stable (1,3-diaxial interactions); tert-butylcyclohexane Disubstituted cyclohexanes: cis/trans-isomerism; comparison of stability (the fewer are the axial substituents, the more stable is the disubstituted cyclohexane. Fused rings (cis ...
Synthetic Transformations of C=O Compounds Reaction Summary
... o Reacts with α,β-unsaturated aldehydes and ketones to give β-substituted carbonyl compounds. This process is called 1,4-addition or conjugate addition. O R ...
... o Reacts with α,β-unsaturated aldehydes and ketones to give β-substituted carbonyl compounds. This process is called 1,4-addition or conjugate addition. O R ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.