How to study organic chemistry?
... Which type of systems act as E+ or Nu-? Same system act as E+ or Nu- depending on which system it reacts. Acidity and Basicity: General idea of order of acidity and basicity. Bronsted & Lewis theory. Effect of back-bonding , aromaticity, SIR,etc. ...
... Which type of systems act as E+ or Nu-? Same system act as E+ or Nu- depending on which system it reacts. Acidity and Basicity: General idea of order of acidity and basicity. Bronsted & Lewis theory. Effect of back-bonding , aromaticity, SIR,etc. ...
Bulent Terem - CH324 - Syllabus | Chaminade
... This is the second part of a two-semester course in organic chemistry. It is assumed that by now the participants have a sound understanding of the basic concepts of organic reaction mechanisms. In the next sixteen weeks we will start with topics in physical organic chemistry and gradually move into ...
... This is the second part of a two-semester course in organic chemistry. It is assumed that by now the participants have a sound understanding of the basic concepts of organic reaction mechanisms. In the next sixteen weeks we will start with topics in physical organic chemistry and gradually move into ...
final1-final_report
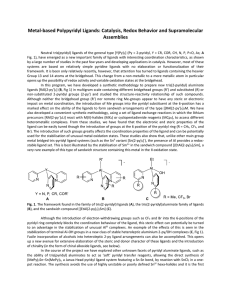
... Neutral tris(pyridyl) ligands of the general type [Y(Py) 3] (Py = 2-pyridyl, Y = CR, COR, CH, N, P, P=O, As; A Fig. 1), have emerged as a new important family of ligands with interesting coordination characteristics, as shown by a large number of studies in the past few years and developing applicat ...
... Neutral tris(pyridyl) ligands of the general type [Y(Py) 3] (Py = 2-pyridyl, Y = CR, COR, CH, N, P, P=O, As; A Fig. 1), have emerged as a new important family of ligands with interesting coordination characteristics, as shown by a large number of studies in the past few years and developing applicat ...
Formative 3.5 2014
... (ii) Amino acids can form polymers because at each end of the molecule is a functional group that can react with a functional group from neighbouring molecules. (b) To be able to form enantiomers a molecule must have a chiral atom – one to which four different groups are attached. This enables the f ...
... (ii) Amino acids can form polymers because at each end of the molecule is a functional group that can react with a functional group from neighbouring molecules. (b) To be able to form enantiomers a molecule must have a chiral atom – one to which four different groups are attached. This enables the f ...
review sheet plus practice problems
... SN2 reaction of an unhindered alkyl halide R-X with OH- to form the alcohol R-OH Reduction of carbonyl compounds: NaBH4 (ald/ketones), LiAlH4 (ald/ketones/esters) **Addition of Grignards (RMgX) or R-Li to aldehydes and ketones: mechanism, limitations. Reactions of alcohols: Deprotonation with strong ...
... SN2 reaction of an unhindered alkyl halide R-X with OH- to form the alcohol R-OH Reduction of carbonyl compounds: NaBH4 (ald/ketones), LiAlH4 (ald/ketones/esters) **Addition of Grignards (RMgX) or R-Li to aldehydes and ketones: mechanism, limitations. Reactions of alcohols: Deprotonation with strong ...
chemistry_23 - Bonar Law Memorial
... Have you heard of benzaldehyde or vanillin? It is likely that you have eaten these organic molecules, called aldehydes, in ice cream or cookies. You will read about the properties that are associated with carbonyl compounds, such as aldehydes. Aldehydes and Ketones What is the structure of a carbony ...
... Have you heard of benzaldehyde or vanillin? It is likely that you have eaten these organic molecules, called aldehydes, in ice cream or cookies. You will read about the properties that are associated with carbonyl compounds, such as aldehydes. Aldehydes and Ketones What is the structure of a carbony ...
I (21 points) Complete the following reactions by providing starting
... A. (JOC, 2008, ASAP, Loh) Chemists have been studying the Barbier-Grignard reactions with the goal of affecting the carbon-carbon bond forming reaction in solvents like water. Recent developments include the use of indium metal catalysts that react through single electron transfer mechanisms. Show t ...
... A. (JOC, 2008, ASAP, Loh) Chemists have been studying the Barbier-Grignard reactions with the goal of affecting the carbon-carbon bond forming reaction in solvents like water. Recent developments include the use of indium metal catalysts that react through single electron transfer mechanisms. Show t ...
Powerpoint File - University of Evansville Faculty Web sites
... water-based life. Note that many biological molecules have two or more functional groups (e.g., amino acids - contain at least one ...
... water-based life. Note that many biological molecules have two or more functional groups (e.g., amino acids - contain at least one ...
Organic Functional Groups Organic Functional Groups
... AMINES ARE NOT AMIDES! • Amines do not have a carbonyl group directly attached to ...
... AMINES ARE NOT AMIDES! • Amines do not have a carbonyl group directly attached to ...
Notes 07 Organometallic Compounds
... Creation of new C-C bonds. ______________are best, otherwise an elimination reaction can occur. The R’ group in the halide can be ______________ The R group of the cuprate can be ______________ Although the mechanism looks like a _________ reaction, it is more complex and is not well understood. ...
... Creation of new C-C bonds. ______________are best, otherwise an elimination reaction can occur. The R’ group in the halide can be ______________ The R group of the cuprate can be ______________ Although the mechanism looks like a _________ reaction, it is more complex and is not well understood. ...
Chapter 9. Addition Reactions of Alkenes
... The reaction below, which provides compound M as its major product, appears to defy the principles that we discussed in class. Draw the structures of the intermediate carbocations that form in this reaction, then clearly but briefly explain why M, and not L, is the major product of this reaction. Hi ...
... The reaction below, which provides compound M as its major product, appears to defy the principles that we discussed in class. Draw the structures of the intermediate carbocations that form in this reaction, then clearly but briefly explain why M, and not L, is the major product of this reaction. Hi ...
- EdShare - University of Southampton
... Alkenes are unsaturated compounds that can be used in organic synthesis. They can be formed in elimination reactions of halogenoalkanes. An example of this is the reaction between 2-bromopentane and hot ethanolic KOH. Using your knowledge of reaction mechanisms, draw appropriate curly arrows to comp ...
... Alkenes are unsaturated compounds that can be used in organic synthesis. They can be formed in elimination reactions of halogenoalkanes. An example of this is the reaction between 2-bromopentane and hot ethanolic KOH. Using your knowledge of reaction mechanisms, draw appropriate curly arrows to comp ...
CN>Chapter 22CT>Carbonyl Alpha
... Many schemes make use of carbonyl substitution reactions. These reaction are one the few general methods for making C-C bonds. ...
... Many schemes make use of carbonyl substitution reactions. These reaction are one the few general methods for making C-C bonds. ...
n - TU Chemnitz
... a) S. Kirchmeyer, A. Mertens, G. A. Olah, Synthesis 1983, 500–502; b) A. Hassner, R. Fibiger, A. S. Amarasekara, J. Org. Chem. 1988, 53, 22–27. a) P. Herczegh, M. Zsély, I. Kovács, G. Batta, F. J. Sztaricskai, Tetrahedron Lett. 1990, 31, 1195–1198. b) C. Gauthier, Y. Ramondenc, G. Plé, ...
... a) S. Kirchmeyer, A. Mertens, G. A. Olah, Synthesis 1983, 500–502; b) A. Hassner, R. Fibiger, A. S. Amarasekara, J. Org. Chem. 1988, 53, 22–27. a) P. Herczegh, M. Zsély, I. Kovács, G. Batta, F. J. Sztaricskai, Tetrahedron Lett. 1990, 31, 1195–1198. b) C. Gauthier, Y. Ramondenc, G. Plé, ...
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to
... t Attack of carbanion-like ylide on the carbonyl leads to formation of ...
... t Attack of carbanion-like ylide on the carbonyl leads to formation of ...
Practice Questions Survey II – 1152 1. The bond angles around the
... a. the presence of one or more carbon-carbon double bonds b. the presence of one or more carbon-carbon triple bonds c. the presence of at least one carbon-carbon double bond, and at least one carbon-carbon triple bond d. the presence of a ring system 19. Given an alkane, an alkene, and an alkyne, ea ...
... a. the presence of one or more carbon-carbon double bonds b. the presence of one or more carbon-carbon triple bonds c. the presence of at least one carbon-carbon double bond, and at least one carbon-carbon triple bond d. the presence of a ring system 19. Given an alkane, an alkene, and an alkyne, ea ...
Aldehydes and Ketones Both contain the functional group C O
... Of the two reducing agents, sodium borohydride is the milder reagent and is the one of preference for aldehydes and ketones since it is specific for these two functional groups. Lithium aluminum hydride will reduce many types of compounds very quickly. Reduction to hydrocarbons (we saw this used to ...
... Of the two reducing agents, sodium borohydride is the milder reagent and is the one of preference for aldehydes and ketones since it is specific for these two functional groups. Lithium aluminum hydride will reduce many types of compounds very quickly. Reduction to hydrocarbons (we saw this used to ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.