J. Indian. Chem. Soc. 1999, 76, 631-639
... N-protected amino acid allylic esters can easily be deprotonated by LDA at -78 °C and transmetallated by addition of metal salts. Chelated metal enolates, which undergo Claisen rearrangements upon warming up to room temperature, giving rise to unsaturated amino acids, are formed with many different ...
... N-protected amino acid allylic esters can easily be deprotonated by LDA at -78 °C and transmetallated by addition of metal salts. Chelated metal enolates, which undergo Claisen rearrangements upon warming up to room temperature, giving rise to unsaturated amino acids, are formed with many different ...
A NEW APROACH TO N-SUBSTITUTED OXAZOLIDINE VIA NITRILIUM ION TRAPPING
... Abstract: We have recently demonstrated a direct conversion of secondary alcohols to amides with retention of configuration. This method involved the in situ formation of chlorosulfites followed by a reaction with nitrile complexes of Ti(IV) fluoride. We hypothesize that these amidation reactions in ...
... Abstract: We have recently demonstrated a direct conversion of secondary alcohols to amides with retention of configuration. This method involved the in situ formation of chlorosulfites followed by a reaction with nitrile complexes of Ti(IV) fluoride. We hypothesize that these amidation reactions in ...
C h e m g u id e –... ACID ANHYDRIDES: INTRODUCTION
... b) It reacts with moisture in the air or your nose to produce ethanoic acid. c) Ethanoic anhydride reacts with water and doesn’t simply dissolve in it. 2. Ethanoyl chloride reacts with molecules containing a hydrogen atom attached to the electronegative atoms oxygen and nitrogen – for example water, ...
... b) It reacts with moisture in the air or your nose to produce ethanoic acid. c) Ethanoic anhydride reacts with water and doesn’t simply dissolve in it. 2. Ethanoyl chloride reacts with molecules containing a hydrogen atom attached to the electronegative atoms oxygen and nitrogen – for example water, ...
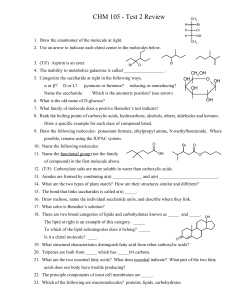
CHM 105 - Test 2 Review
... of compound) in the first molecule above. 12. (T/F) Carboxylate salts are more soluble in water than carboxylic acids. 13. Amides are formed by combining a(n) _____________ and a(n) __________________________. 14. What are the two types of plant starch? How are their structures similar and different ...
... of compound) in the first molecule above. 12. (T/F) Carboxylate salts are more soluble in water than carboxylic acids. 13. Amides are formed by combining a(n) _____________ and a(n) __________________________. 14. What are the two types of plant starch? How are their structures similar and different ...
Solution 1. - TutorBreeze.com
... (iv) Aldol :- An aldol or aldol adduct is a beta-hydroxy ketone or aldehyde, and is the product of aldol condensation, in which two molecules of aldehydes , or two molecules of ketones or one molecule of aldehyde and one molecule of ketone condense in the presence of aqueos sodium hydroxide to form ...
... (iv) Aldol :- An aldol or aldol adduct is a beta-hydroxy ketone or aldehyde, and is the product of aldol condensation, in which two molecules of aldehydes , or two molecules of ketones or one molecule of aldehyde and one molecule of ketone condense in the presence of aqueos sodium hydroxide to form ...
Abstracts - Thieme Verlag
... Hydroformylation of alkenes is a mild and clean method for the functionalization of hydrocarbons and has grown to be among the most important homogeneously catalyzed reactions in industry. It is a 100% atom-economic reaction to prepare aldehydes that are important synthons for organic synthesis, and ...
... Hydroformylation of alkenes is a mild and clean method for the functionalization of hydrocarbons and has grown to be among the most important homogeneously catalyzed reactions in industry. It is a 100% atom-economic reaction to prepare aldehydes that are important synthons for organic synthesis, and ...
Microsoft Word
... to amino group, or nucleophilic displacement. The resulting β-hydroxy nitro compounds have been used in various beneficial transformations to provide chiral β-amino alcohols and α-hydroxy carboxylic acids. Attention has recently been focused on the development of catalytic, asymmetric versions of th ...
... to amino group, or nucleophilic displacement. The resulting β-hydroxy nitro compounds have been used in various beneficial transformations to provide chiral β-amino alcohols and α-hydroxy carboxylic acids. Attention has recently been focused on the development of catalytic, asymmetric versions of th ...
Chapter 14 Notes
... • Using the root alkane name, drop the –e ending and change to –one. • Number the longest carbon chain so the C=O group has the lowest number. • Name and number other substituents as before. Examples: ...
... • Using the root alkane name, drop the –e ending and change to –one. • Number the longest carbon chain so the C=O group has the lowest number. • Name and number other substituents as before. Examples: ...
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the
... The parent chain is numbered to give the ketone carbonyl the lowest possible number In common nomenclature simple ketones are named by preceding the word ketone with the names of both groups attached to the ketone carbonyl ...
... The parent chain is numbered to give the ketone carbonyl the lowest possible number In common nomenclature simple ketones are named by preceding the word ketone with the names of both groups attached to the ketone carbonyl ...
Biochemistry Review
... preventing the enzyme from working, are considered “negative” and result in noncompetitive inhibition. If a substance, in either of these cases, is an end product of the reactions brought on by the enzyme, than feedback inhibition occurs, a selfregulating system that stops the reaction when enough ...
... preventing the enzyme from working, are considered “negative” and result in noncompetitive inhibition. If a substance, in either of these cases, is an end product of the reactions brought on by the enzyme, than feedback inhibition occurs, a selfregulating system that stops the reaction when enough ...
C - Milwaukie High
... a main carbon backbone AND one or more branches Substituted compounds have… one or more H atoms removed, with other atoms in their place(s) (often halogens) ...
... a main carbon backbone AND one or more branches Substituted compounds have… one or more H atoms removed, with other atoms in their place(s) (often halogens) ...
Oxidative Addition
... When the incoming ligand is 13CO, the product contains only one labeled CO, which is cis to the newly formed acetyl group. This shows that the methyl group migrates to a coordinated CO, rather than free CO attacking the Mn−Me bond. We can tell where the labeled CO is located in the product becau ...
... When the incoming ligand is 13CO, the product contains only one labeled CO, which is cis to the newly formed acetyl group. This shows that the methyl group migrates to a coordinated CO, rather than free CO attacking the Mn−Me bond. We can tell where the labeled CO is located in the product becau ...
Chapter 4-Carbon & Diversity of Life
... Carbon has four bonding sites This allows for large and complex molecules to be made with this element They may form flat or tetrahedral molecules and may also form rings, chains or branched molecules Carbon may also bond with itself as well as other common elements like Nitrogen, Hydrogen, and Oxyg ...
... Carbon has four bonding sites This allows for large and complex molecules to be made with this element They may form flat or tetrahedral molecules and may also form rings, chains or branched molecules Carbon may also bond with itself as well as other common elements like Nitrogen, Hydrogen, and Oxyg ...
Chapter 5 – Chemical Reactions
... reaction will be a single replacement. A more reactive metal will replace a less reactive metal or hydrogen. The more reactive metals are located near Cs and Fr on the chart. A more reactive non-metal will replace a less reactive non-metal. The more reactive non-metals are near F on the chart. ...
... reaction will be a single replacement. A more reactive metal will replace a less reactive metal or hydrogen. The more reactive metals are located near Cs and Fr on the chart. A more reactive non-metal will replace a less reactive non-metal. The more reactive non-metals are near F on the chart. ...
Alkanes
... What is the general formula for a dihalogenation reaction? CnH2n+2 + 2X2 CnH2nX2 + 2HX ...
... What is the general formula for a dihalogenation reaction? CnH2n+2 + 2X2 CnH2nX2 + 2HX ...
4.5 Topic Checklist Carbonyl Compounds
... a recognition of the properties and uses of acids and esters. an appreciation of the use of acid chlorides in organic synthesis. an appreciation of how acid chlorides may react at a molecular level. a series of practical skills associated with the synthesis and analysis of organic compounds. ...
... a recognition of the properties and uses of acids and esters. an appreciation of the use of acid chlorides in organic synthesis. an appreciation of how acid chlorides may react at a molecular level. a series of practical skills associated with the synthesis and analysis of organic compounds. ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.