* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Topic: Function Group #4
Survey
Document related concepts
Transcript
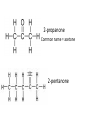
Topic: Function Group #4-Ketones Do Now: In terms of IMF, explain the difference in boiling point O = • General Format: RCR' R and R' may or may not be the same (but have to be carbons/carbon chains) Carbonyl group: = O C located within C chain Properties • Carboxyl group makes ketone molecules polar = soluble in water • Dipole-Dipole interactions – Boiling point: • higher than alkanes (same # C’s) • lower than alcohols (same # C’s) Have you ever smelled a ketone?! Camphor Naming Ketones • Must use number to indicate location of carboxyl group – use lowest possible number • Take corresponding alkane name: – Drop -e & add -one 2-propanone Common name = acetone 2-pentanone