Chapter 11 Chemical Reactions
... arrow (→) separates the reactants from the products (arrow points to products) –Read as: “reacts to form” or yields The plus sign = “and” (s) after the formula = solid: Fe(s) (g) after the formula = gas: CO2(g) (l) after the formula = liquid: H2O(l) ...
... arrow (→) separates the reactants from the products (arrow points to products) –Read as: “reacts to form” or yields The plus sign = “and” (s) after the formula = solid: Fe(s) (g) after the formula = gas: CO2(g) (l) after the formula = liquid: H2O(l) ...
Grignard Reactions - faculty at Chemeketa
... reagents in organic chemistry. We will consider only its reactions with aldehydes and ketones at this time. Grignards react with aldehydes and ketones to give intermediate products that form alcohols when hydrolyzed. With formaldehyde, primary alcohols are formed; with other aldehydes, secondary alc ...
... reagents in organic chemistry. We will consider only its reactions with aldehydes and ketones at this time. Grignards react with aldehydes and ketones to give intermediate products that form alcohols when hydrolyzed. With formaldehyde, primary alcohols are formed; with other aldehydes, secondary alc ...
1 Chapter 8: Nucleophilic Substitution 8.1: Functional Group
... formation must involve both reactants and explain the stereospecificity . ...
... formation must involve both reactants and explain the stereospecificity . ...
Name - rwebbchem
... ____________________________________________________________ Type of Reaction: ___________________________ Day 10: ___________ Solubility Rules: Use the solubility rules to complete the following: 1. Would a precipitate form from a reaction of aluminum chloride and sodium hydroxide? If yes, write an ...
... ____________________________________________________________ Type of Reaction: ___________________________ Day 10: ___________ Solubility Rules: Use the solubility rules to complete the following: 1. Would a precipitate form from a reaction of aluminum chloride and sodium hydroxide? If yes, write an ...
Chapter 12 and 13 Notes
... Solubility is less than that for similar number of C’s with alcohol group. Larger ones are not soluble. Oxidation of aldehydes and ketones is like that for the alcohol series. ...
... Solubility is less than that for similar number of C’s with alcohol group. Larger ones are not soluble. Oxidation of aldehydes and ketones is like that for the alcohol series. ...
Unit - III - E
... controlling where and from what direction a molecular interaction can take place. ...
... controlling where and from what direction a molecular interaction can take place. ...
CHEMICAL REACTIONS OBJECTIVES 1. To study reactions
... 1. The unbalanced molecular chemical equations for the 4 reactions in Part I A are given below. Write the balanced molecular equations for each. Be sure to indicate the state of each product [(s) for solid, (l) for liquid, (g) for gas, (aq) for aqueous]. You may need to refer to the list of solubili ...
... 1. The unbalanced molecular chemical equations for the 4 reactions in Part I A are given below. Write the balanced molecular equations for each. Be sure to indicate the state of each product [(s) for solid, (l) for liquid, (g) for gas, (aq) for aqueous]. You may need to refer to the list of solubili ...
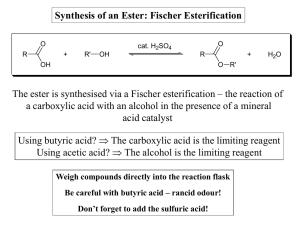
Synthesis of an Ester: Fischer Esterification The ester is synthesised
... The yield of the reaction is also dependent on the position of the equilibrium (i.e. it not lying completely in favour of products) A number of methods can be employed to drive the reaction completely to product, including: - Removing one of the products (ester or water) from the reaction, as it is ...
... The yield of the reaction is also dependent on the position of the equilibrium (i.e. it not lying completely in favour of products) A number of methods can be employed to drive the reaction completely to product, including: - Removing one of the products (ester or water) from the reaction, as it is ...
Chapter 4 Stereochemistry and Chirality Flow chart for determining
... Enantiotopic: If you have a methylene group of the form X- CH2 -Z, and you replace one of the hydrogens of the CH2 with a dummy atom and then you independently replace the other hydrogen of the CH 2 group with a dummy atom (Y), the two molecules thus created will be enantiomers of each other. The pr ...
... Enantiotopic: If you have a methylene group of the form X- CH2 -Z, and you replace one of the hydrogens of the CH2 with a dummy atom and then you independently replace the other hydrogen of the CH 2 group with a dummy atom (Y), the two molecules thus created will be enantiomers of each other. The pr ...
CN>Chapter 22CT>Carbonyl Alpha
... as being in the α position Electrophilic substitution occurs at this position through either an enol or enolate ion ...
... as being in the α position Electrophilic substitution occurs at this position through either an enol or enolate ion ...
Elimination Reactions
... In this, two-step process, the rate of the reaction is dependent on the rate of ionization of the substrate (as was the case in the SN1 reaction) ...
... In this, two-step process, the rate of the reaction is dependent on the rate of ionization of the substrate (as was the case in the SN1 reaction) ...
Microsoft Word - Open Access Repository of Indian Theses
... Jacobson’s asymmetric epoxidation followed by ring expansion. Although these methods are fairly efficient (e.e.94-99%, yields 65-75%), they require expensive chiral ligands to induce chirality or highly toxic osmium complexes. Other strategies involve intermediates derived from amino acids such as L ...
... Jacobson’s asymmetric epoxidation followed by ring expansion. Although these methods are fairly efficient (e.e.94-99%, yields 65-75%), they require expensive chiral ligands to induce chirality or highly toxic osmium complexes. Other strategies involve intermediates derived from amino acids such as L ...
Reductions of Carboxylic Acid Derivatives - IDC
... for the reduction of aldehydes and ketones to 1º and 2º-alcohols respectively has been noted. Of these, lithium aluminum hydride, often abbreviated LAH, is the most useful for reducing carboxylic acid derivatives. Thanks to its high reactivity, LAH easily reduces all classes of carboxylic acid deriv ...
... for the reduction of aldehydes and ketones to 1º and 2º-alcohols respectively has been noted. Of these, lithium aluminum hydride, often abbreviated LAH, is the most useful for reducing carboxylic acid derivatives. Thanks to its high reactivity, LAH easily reduces all classes of carboxylic acid deriv ...
Kazzie`s Guide to Orgo 2
... Guide to Orgo 2 General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 21 ...
... Guide to Orgo 2 General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 21 ...
Cell Molecules
... hydrogen atom and hydroxyl group from a split water molecule attaches where the covalent bond used to be. – Hydrolysis reactions dominate the digestive process, guided by specific enzymes. ...
... hydrogen atom and hydroxyl group from a split water molecule attaches where the covalent bond used to be. – Hydrolysis reactions dominate the digestive process, guided by specific enzymes. ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.