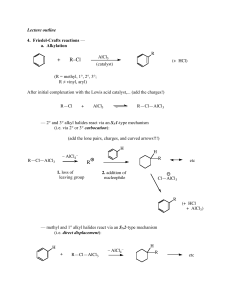
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 PART-A
... 11. Give an example for α, β, γ and δ-elimination reaction. 12. State and explain the Hammond postulate to the bromination of n-propane. 13. How will you determine the reaction mechanism of hydrolysis of an ester using ...
... 11. Give an example for α, β, γ and δ-elimination reaction. 12. State and explain the Hammond postulate to the bromination of n-propane. 13. How will you determine the reaction mechanism of hydrolysis of an ester using ...
Chapter 11 Lecture Notes: Alcohols, Ethers, Aldehydes, and Ketones
... group and an -OH group that are bonded to the same carbon. Carbons that are bonded to both an -OR group and an -OH group are called hemiacetal carbons. Carbon number 1 in the ring structure shown meets this criterion. The OH that is bonded to carbon number 1 is obvious, but the OR may not be immedia ...
... group and an -OH group that are bonded to the same carbon. Carbons that are bonded to both an -OR group and an -OH group are called hemiacetal carbons. Carbon number 1 in the ring structure shown meets this criterion. The OH that is bonded to carbon number 1 is obvious, but the OR may not be immedia ...
Exam 3 - Organic Chemistry at CU Boulder
... Appendix: Periodic Table, common NMR, IR absorption ...
... Appendix: Periodic Table, common NMR, IR absorption ...
Chapter 2 Chemical Reactions
... Rules for balancing: 1) Assemble the correct formulas for all the reactants and products, using “+” and “→” 2) Count the number of atoms of each type appearing on both sides 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the ...
... Rules for balancing: 1) Assemble the correct formulas for all the reactants and products, using “+” and “→” 2) Count the number of atoms of each type appearing on both sides 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the ...
ch13[1].
... The Carbonyl Group • In this and several following chapters, we study the physical and chemical properties of classes of compounds containing the carbonyl group, C=O. • aldehydes and ketones (Chapter 13) • carboxylic acids (Chapter 14) • acid halides, acid anhydrides, esters, and amides ...
... The Carbonyl Group • In this and several following chapters, we study the physical and chemical properties of classes of compounds containing the carbonyl group, C=O. • aldehydes and ketones (Chapter 13) • carboxylic acids (Chapter 14) • acid halides, acid anhydrides, esters, and amides ...
Topic Selection Menu - Pennsylvania State University
... • Formation of achiral carbocation intermediate • Pro-R face, pro-S face – Reaction profile energy diagram – Worked Examples ...
... • Formation of achiral carbocation intermediate • Pro-R face, pro-S face – Reaction profile energy diagram – Worked Examples ...
Solvent free permanganate oxidations
... may make it the approach of choice. When the a-carbons are tertiary, the product is a dione, formed presumably by dehydration of the corresponding diol as in Scheme 6. Addition of alumina to the solid support did not improve the yields of these reactions. Arenes are converted into the corresponding ...
... may make it the approach of choice. When the a-carbons are tertiary, the product is a dione, formed presumably by dehydration of the corresponding diol as in Scheme 6. Addition of alumina to the solid support did not improve the yields of these reactions. Arenes are converted into the corresponding ...
Lecture: 2 OCCURRENCE AND STRUCTURE OF
... Commercially sorbitol is manufactured by the hydrogenation of glucose. Mannitol occurs in many terrestrial and marine plants. Potential food applications of polyols include confectionery products, bakery products, deserts, jams and marmalade. ...
... Commercially sorbitol is manufactured by the hydrogenation of glucose. Mannitol occurs in many terrestrial and marine plants. Potential food applications of polyols include confectionery products, bakery products, deserts, jams and marmalade. ...
2 - DrChoChemistryWebSite
... For some, we will be able to: c) predict whether or not they will happen at all. ...
... For some, we will be able to: c) predict whether or not they will happen at all. ...
06_reactions
... (there will be more reactants or products depending on half-reactions) Example: methanal + Tollens’ reagent → methanoic acid + silver + ammonia CH2O + Ag(NH3)2+ + H2O → CHOOH + Ag + 2NH3 + 2H+ Since an obvious silver ‘mirror’ forms on the flask if a reaction occurs, this is commonly used to test whe ...
... (there will be more reactants or products depending on half-reactions) Example: methanal + Tollens’ reagent → methanoic acid + silver + ammonia CH2O + Ag(NH3)2+ + H2O → CHOOH + Ag + 2NH3 + 2H+ Since an obvious silver ‘mirror’ forms on the flask if a reaction occurs, this is commonly used to test whe ...
Alcohols
... Aldehydes and ketones are polar molecules because of the polar carbon-oxygen double bond. Their boiling points are lower than alcohols of similar sizes because the do not form hydrogen bonds. They are more soluble in water then hydrocarbons. These compounds are soluble in both polar and nonp ...
... Aldehydes and ketones are polar molecules because of the polar carbon-oxygen double bond. Their boiling points are lower than alcohols of similar sizes because the do not form hydrogen bonds. They are more soluble in water then hydrocarbons. These compounds are soluble in both polar and nonp ...
Single-Replacement Reactions
... Balance the atoms of an element one at a time by adding coefficients (the numbers in front) - save H and O until LAST! Check to make sure it is balanced. ...
... Balance the atoms of an element one at a time by adding coefficients (the numbers in front) - save H and O until LAST! Check to make sure it is balanced. ...
Chapter 9-Additions to Alkenes I
... according to the Hammond postulate, any factor that stabilizes a high energy intermediate should also stabilize the transition state leading to that intermediate. So, factors that lead to a more stable carbocation intermediate will also stabilize the transition state of HX addition to alkene! Suppos ...
... according to the Hammond postulate, any factor that stabilizes a high energy intermediate should also stabilize the transition state leading to that intermediate. So, factors that lead to a more stable carbocation intermediate will also stabilize the transition state of HX addition to alkene! Suppos ...
Reductive Coupling Reactions of Nitrones and Imines
... This conformational stability inhibits bond rotation about the ferrocene-C=N bond, providing the basis for facial selectivity imposed by the steric bulk of the ferrocene ring systems. The imine dianion and the carbonyl group align to minimize dipole-dipole repulsion, resulting in the observed anti d ...
... This conformational stability inhibits bond rotation about the ferrocene-C=N bond, providing the basis for facial selectivity imposed by the steric bulk of the ferrocene ring systems. The imine dianion and the carbonyl group align to minimize dipole-dipole repulsion, resulting in the observed anti d ...
CHE 106, F`95 E1(Word)
... The sum of the masses of two hydrogen atoms (mass number 1) and two neutrons is 4.0330. Why does this differ from the mass of a helium atom (4.0026)? (a) (b) (c) (d) (e) ...
... The sum of the masses of two hydrogen atoms (mass number 1) and two neutrons is 4.0330. Why does this differ from the mass of a helium atom (4.0026)? (a) (b) (c) (d) (e) ...
Enzymes
... orientation for the reaction. As the active site binds the substrate, it may put stress on bonds that must be broken, making it easier to reach the transition state. R groups at the active site may create a conducive microenvironment for a specific reaction. Enzymes may even bind covalently to subst ...
... orientation for the reaction. As the active site binds the substrate, it may put stress on bonds that must be broken, making it easier to reach the transition state. R groups at the active site may create a conducive microenvironment for a specific reaction. Enzymes may even bind covalently to subst ...
Chapter 11 Chemical Reactions
... For some, we will be able to: c) predict whether or not they will happen at all. ...
... For some, we will be able to: c) predict whether or not they will happen at all. ...
Chemical Reactions
... Never change a subscript to balance an equation (You can only change coefficients) – If you change the subscript (formula) you are describing a different chemical. – H2O is a different compound than H2O2 Never put a coefficient in the middle of a formula; they must go only in the front ...
... Never change a subscript to balance an equation (You can only change coefficients) – If you change the subscript (formula) you are describing a different chemical. – H2O is a different compound than H2O2 Never put a coefficient in the middle of a formula; they must go only in the front ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.



![ch13[1].](http://s1.studyres.com/store/data/008194698_1-d188c504eac7b7806e762a2340484910-300x300.png)