Cell Molecules
... hydrogen atom and hydroxyl group from a split water molecule attaches where the covalent bond used to be. – Hydrolysis reactions dominate the digestive process, guided by specific enzymes. ...
... hydrogen atom and hydroxyl group from a split water molecule attaches where the covalent bond used to be. – Hydrolysis reactions dominate the digestive process, guided by specific enzymes. ...
Chapter 12: IR Spectroscopy and Mass Spectrometry
... band is normally the strongest one in the spectrum. The normal carbonyl absorption is around 1710-1720 cm-1 ...
... band is normally the strongest one in the spectrum. The normal carbonyl absorption is around 1710-1720 cm-1 ...
ALDEHYDES , KETONES AND CARBOXYLIC ACIDS
... • The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers due to dipole dipoe interaction and lower than those of alcohols due to absence of intermolecular hydrogen bonding. • The lower members of aldehydes and ketones are miscible with water due to hydrogen bonding. 1. C ...
... • The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers due to dipole dipoe interaction and lower than those of alcohols due to absence of intermolecular hydrogen bonding. • The lower members of aldehydes and ketones are miscible with water due to hydrogen bonding. 1. C ...
CHEMICAL REACTIONS
... (s) after the formula –solid Cu(s) (g) after the formula –gas H2 (g) (l) after the formula -liquid H2O(l) (aq) after the formula - dissolved in water, an aqueous solution. CaCl2 (aq) used after a product indicates a gas (same as (g)) O2 used after a product indicates a solid (same as (s)) ...
... (s) after the formula –solid Cu(s) (g) after the formula –gas H2 (g) (l) after the formula -liquid H2O(l) (aq) after the formula - dissolved in water, an aqueous solution. CaCl2 (aq) used after a product indicates a gas (same as (g)) O2 used after a product indicates a solid (same as (s)) ...
Nucleophilic Substitution Reactions
... electronegative than carbon, and so exserts a stronger pull on the shared electrons in the carbon-halogen bond. ■ As a result, the halogen gains a partial negative charge and the carbon gains a partial positive charge, and it is said to be electron deficient. ...
... electronegative than carbon, and so exserts a stronger pull on the shared electrons in the carbon-halogen bond. ■ As a result, the halogen gains a partial negative charge and the carbon gains a partial positive charge, and it is said to be electron deficient. ...
A-level Paper 2 Practice Paper 1 - A
... Cetrimide is used as an antiseptic. [CH3(CH2)15N(CH3)3]+ Br– cetrimide Name this type of compound. Give the reagent that must be added to CH3(CH2)15NH2 to make cetrimide and state the reaction conditions. Name the type of mechanism involved in this reaction. ...
... Cetrimide is used as an antiseptic. [CH3(CH2)15N(CH3)3]+ Br– cetrimide Name this type of compound. Give the reagent that must be added to CH3(CH2)15NH2 to make cetrimide and state the reaction conditions. Name the type of mechanism involved in this reaction. ...
Lecture 2 - UCLA Chemistry and Biochemistry
... The product distribution follows more or less the degree of stability of the product because the reaction is carried out under thermodynamic conditions (elevated ...
... The product distribution follows more or less the degree of stability of the product because the reaction is carried out under thermodynamic conditions (elevated ...
Chemistry 21 A - El Camino College
... 2) Does your reaction have two (or more) chemicals combining to form one chemical? If yes, then it's a synthesis reaction 3) Does your reaction have one large molecule falling apart to make several small ones? If yes, then it's a decomposition reaction 4) Does your reaction have any molecules that c ...
... 2) Does your reaction have two (or more) chemicals combining to form one chemical? If yes, then it's a synthesis reaction 3) Does your reaction have one large molecule falling apart to make several small ones? If yes, then it's a decomposition reaction 4) Does your reaction have any molecules that c ...
Monosaccharide
... • Fischer projections depict three-dimensional shapes for chiral molecules, with the chiral carbon represented by the intersection of two lines. ...
... • Fischer projections depict three-dimensional shapes for chiral molecules, with the chiral carbon represented by the intersection of two lines. ...
Functional Groups Notes
... exhibits characteristic and predictable chemical behavior. A particular functional group generally exhibits a particular type of behavior, regardless of the nature of its parent molecule. These functional groups are molecular structural features that allow chemists to classify compounds by their rea ...
... exhibits characteristic and predictable chemical behavior. A particular functional group generally exhibits a particular type of behavior, regardless of the nature of its parent molecule. These functional groups are molecular structural features that allow chemists to classify compounds by their rea ...
Aldehydes and ketones
... A hemiacetal is an organic compound that derives from an aldehyde and possesses a carbon atom that is bound to an OH (hydroxy) group and an OR (alkoxy) group Hemiketal: same as above, but it derives from a ketone, not an aldehyde. ...
... A hemiacetal is an organic compound that derives from an aldehyde and possesses a carbon atom that is bound to an OH (hydroxy) group and an OR (alkoxy) group Hemiketal: same as above, but it derives from a ketone, not an aldehyde. ...
Survey on Conditions Catalysis of Chemical Reactions
... will reduce aldehydes, ketones, esters, carboxylic acid chlorides, carboxylic acids and even carboxylate salts to alcohols. Amides and nitriles are reduced to amines. In each case the partially negative hydrogen reacts with the partially positive carbon of the substrate. It can also be used to reduc ...
... will reduce aldehydes, ketones, esters, carboxylic acid chlorides, carboxylic acids and even carboxylate salts to alcohols. Amides and nitriles are reduced to amines. In each case the partially negative hydrogen reacts with the partially positive carbon of the substrate. It can also be used to reduc ...
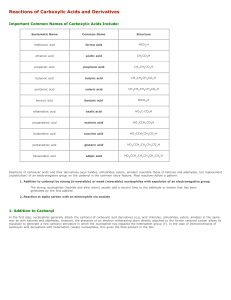
Reactions of Carboxylic Acids and Derivatives
... (substitution) of an electronegative group on the carbonyl is the common extra feature. Most reactions follow a pattern: 1. Addition to carbonyl by strong (irreversible) or weak (reversible) nucleophiles with expulsion of an electronegative group. The strong nucleophiles (hydride and alkyl anion) us ...
... (substitution) of an electronegative group on the carbonyl is the common extra feature. Most reactions follow a pattern: 1. Addition to carbonyl by strong (irreversible) or weak (reversible) nucleophiles with expulsion of an electronegative group. The strong nucleophiles (hydride and alkyl anion) us ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.