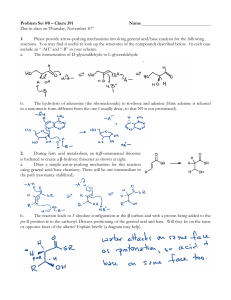
Direct ester condensation catalyzed by bulky diarylammonium
... The ester condensation reaction is among the most fundamental organic transformations, and more environmentally benign alternative synthetic approaches to the ones currently used are in strong demand by the chemical industry1. Conventionally, the ester condensation reaction of carboxylic acids with ...
... The ester condensation reaction is among the most fundamental organic transformations, and more environmentally benign alternative synthetic approaches to the ones currently used are in strong demand by the chemical industry1. Conventionally, the ester condensation reaction of carboxylic acids with ...
Organic Reactions
... a. Alkene + halogen gas 1,2-dihaloalkane b. Diatomic gas has two atoms – both add to opposite sides of the double bond (and opposite sides of the molecule) c. Uses: Chlorine + ethane 1,2-dichloroethane: used as starting material for PVC d. Uses: Br2 dissolved in dichloromethane is used to distin ...
... a. Alkene + halogen gas 1,2-dihaloalkane b. Diatomic gas has two atoms – both add to opposite sides of the double bond (and opposite sides of the molecule) c. Uses: Chlorine + ethane 1,2-dichloroethane: used as starting material for PVC d. Uses: Br2 dissolved in dichloromethane is used to distin ...
enzymatic resolution of a racemic mixture by acylation in
... The synthesis of enantiomerically pure compounds is becoming increasingly important in the pharmaceutical and the fine chemicals industries. The use of enzymes as enantioselective catalysts in kinetic resolutions has now become a common method to obtain pure enantiomers. The applicability of this ap ...
... The synthesis of enantiomerically pure compounds is becoming increasingly important in the pharmaceutical and the fine chemicals industries. The use of enzymes as enantioselective catalysts in kinetic resolutions has now become a common method to obtain pure enantiomers. The applicability of this ap ...
- M E S KVM College Valanchery.
... Dimers of AlMe3 possess delocalized Al–C–Al bonding interactions The bonding in Al2Me4Cl2 can be described in terms of a localized scheme In Al2Ph4(μ-C≡CPh)2, the bridge bonds can be described in a similar way to those in Al2Me4(μ-Ph)2 Al2{CH(SiMe3)2}4 contains an Al–Al bond ...
... Dimers of AlMe3 possess delocalized Al–C–Al bonding interactions The bonding in Al2Me4Cl2 can be described in terms of a localized scheme In Al2Ph4(μ-C≡CPh)2, the bridge bonds can be described in a similar way to those in Al2Me4(μ-Ph)2 Al2{CH(SiMe3)2}4 contains an Al–Al bond ...
Chapter 2.3: Carbon Compounds
... 5. Examples: fats (3 fatty acid chains), phosopholipids (2 fatty acid chains), oils, waxes, ...
... 5. Examples: fats (3 fatty acid chains), phosopholipids (2 fatty acid chains), oils, waxes, ...
Chapter 13. Plannig and Execution of Multistep Synthesis
... Juvabione is terpene-derived keto ester that has been isolated from various plant sources. It exhibits “juvenile hormone” activity in insects; that is, it can modify the process of metamorphosis. ...
... Juvabione is terpene-derived keto ester that has been isolated from various plant sources. It exhibits “juvenile hormone” activity in insects; that is, it can modify the process of metamorphosis. ...
Carbon Compounds
... Draw their structural formulae and write their IUPAC name. Why do alkenes and alkynes burn with sooty flame in air but alkanes burn without soot? State whether alkanes can also burn with sooty flame. If yes, under what conditions? Which of the following will burn with smoke in air: ethane, ethene, p ...
... Draw their structural formulae and write their IUPAC name. Why do alkenes and alkynes burn with sooty flame in air but alkanes burn without soot? State whether alkanes can also burn with sooty flame. If yes, under what conditions? Which of the following will burn with smoke in air: ethane, ethene, p ...
Lecture 15
... 1st Half Reaction: NAD+ + H+ + 2e- --> NADH E°’ = -0.320V 2nd Half Reaction (Note: Its reversed!): FADH2 --> FAD + 2H+ + 2eE°’ = +0.219V E°’= –0.320V + +0.219V ...
... 1st Half Reaction: NAD+ + H+ + 2e- --> NADH E°’ = -0.320V 2nd Half Reaction (Note: Its reversed!): FADH2 --> FAD + 2H+ + 2eE°’ = +0.219V E°’= –0.320V + +0.219V ...
South Pasadena • AP Chemistry
... 11. When H2SO4 and Ba(OH)2 are reacted in a double replacement reaction, one of the products of the reaction is… a) H2 d) BaH2 b) H2O e) SO2 c) BaS 12. In the double replacement reaction between the weak acid, HC2H3O2 and strong base, NaOH, which ion(s) are spectator ions? a) Na+, C2H3O2– d) H+, C2 ...
... 11. When H2SO4 and Ba(OH)2 are reacted in a double replacement reaction, one of the products of the reaction is… a) H2 d) BaH2 b) H2O e) SO2 c) BaS 12. In the double replacement reaction between the weak acid, HC2H3O2 and strong base, NaOH, which ion(s) are spectator ions? a) Na+, C2H3O2– d) H+, C2 ...
6-organic - fixurscore
... Potassium dichromate K2Cr2O7 is an oxidising agent that causes alcohols to oxidise. Partial Oxidation of Primary Alcohols ...
... Potassium dichromate K2Cr2O7 is an oxidising agent that causes alcohols to oxidise. Partial Oxidation of Primary Alcohols ...
Solutions
... reactions. You may find it useful to look up the structures of the compounds described below. In each case include an “-AH” and “-B” in your scheme. a. The isomerization of D-glyceraldehyde to L-glyceraldehyde ...
... reactions. You may find it useful to look up the structures of the compounds described below. In each case include an “-AH” and “-B” in your scheme. a. The isomerization of D-glyceraldehyde to L-glyceraldehyde ...
Metabolic energy from acetic acid catabolism in the hot springs of
... Methanothrix, among others, gain energy from reaction (4). However, at present, no species are known that can harness energy from any of these forms of acetic acid catabolism at temperatures above approximately 55~ This may an artifact of the small number of hyperthermophiles (organisms able to grow ...
... Methanothrix, among others, gain energy from reaction (4). However, at present, no species are known that can harness energy from any of these forms of acetic acid catabolism at temperatures above approximately 55~ This may an artifact of the small number of hyperthermophiles (organisms able to grow ...
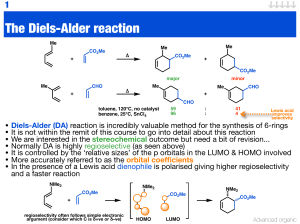
The Diels-Alder reaction
... • Normally DA is highly regioselective (as seen above) • It is controlled by the ‘relative sizes’ of the p orbitals in the LUMO & HOMO involved • More accurately referred to as the orbital coefficients • In the presence of a Lewis acid dienophile is polarised giving higher regioselectivity and a fas ...
... • Normally DA is highly regioselective (as seen above) • It is controlled by the ‘relative sizes’ of the p orbitals in the LUMO & HOMO involved • More accurately referred to as the orbital coefficients • In the presence of a Lewis acid dienophile is polarised giving higher regioselectivity and a fas ...
the suzuki-miyaura reaction and boron reagents – mechanism
... ¤ Not clear what the active transmetalating species is during the SuzukiMiyaura coupling. ...
... ¤ Not clear what the active transmetalating species is during the SuzukiMiyaura coupling. ...
Ester - SCH4U-SRB
... Esters are responsible for the “fruity” odours and flavours of many naturally occurring products. Chemists can reproduce these odours or flavours in the lab by mixing the right alcohol and carboxylic acid together; thus, producing “artificial” or “synthetic” versions of these naturally occurring com ...
... Esters are responsible for the “fruity” odours and flavours of many naturally occurring products. Chemists can reproduce these odours or flavours in the lab by mixing the right alcohol and carboxylic acid together; thus, producing “artificial” or “synthetic” versions of these naturally occurring com ...
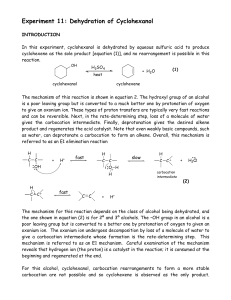
Dehydration of Cyclohexanol
... carbocations derived from certain 2°alcohols may undergo rearrangement to form more stable carbocations. This can result in the formation of rearranged isomeric alkenes. Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symm ...
... carbocations derived from certain 2°alcohols may undergo rearrangement to form more stable carbocations. This can result in the formation of rearranged isomeric alkenes. Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symm ...
DETERMINING THE CONCENTRATION OF A SOLUTION:
... 3. _____ Carefully add 2 - 3 drops of concentrated sulfuric acid (H2SO4) to each test tube. CAUTION: Handle concentrated sulfuric acid carefully. If the acid spills on your skin or clothing, immediately rinse the affected area with water and inform your teacher. 4. _____ Stir each mixture slightly b ...
... 3. _____ Carefully add 2 - 3 drops of concentrated sulfuric acid (H2SO4) to each test tube. CAUTION: Handle concentrated sulfuric acid carefully. If the acid spills on your skin or clothing, immediately rinse the affected area with water and inform your teacher. 4. _____ Stir each mixture slightly b ...
Edexcel GCE - The Student Room
... (c) Lithium can react with chlorine to produce lithium chloride. When a sample of lithium chloride is heated in a Bunsen flame, a red colour is seen. (i) Draw a ‘dot and cross’ diagram of lithium chloride showing all the electrons. Indicate the charges clearly on your diagram. ...
... (c) Lithium can react with chlorine to produce lithium chloride. When a sample of lithium chloride is heated in a Bunsen flame, a red colour is seen. (i) Draw a ‘dot and cross’ diagram of lithium chloride showing all the electrons. Indicate the charges clearly on your diagram. ...
PPT
... • Low molecular weight carboxylic acids are liquids at room temperature and have characteristically sharp or unpleasant odors. • The –COOH group is very polar. Hydrogen bonding between –COOH groups creates dimers (two identical molecules bonded together). ...
... • Low molecular weight carboxylic acids are liquids at room temperature and have characteristically sharp or unpleasant odors. • The –COOH group is very polar. Hydrogen bonding between –COOH groups creates dimers (two identical molecules bonded together). ...
Arginine- or Lysine-catalyzed Michael Addition of Nitromethane to α
... addition of nitroalkanes provide a variety of key building blocks for various compounds, such as an amine, ketone, alkane, and alkene.4 The most widely studied amino acid as a catalyst is proline. The reaction mechanisms of the proline-catalyzed reactions with carbonyl compounds are mainly involved ...
... addition of nitroalkanes provide a variety of key building blocks for various compounds, such as an amine, ketone, alkane, and alkene.4 The most widely studied amino acid as a catalyst is proline. The reaction mechanisms of the proline-catalyzed reactions with carbonyl compounds are mainly involved ...
Word - chemmybear.com
... 11. When H2SO4 and Ba(OH)2 are reacted in a double replacement reaction, one of the products of the reaction is… a) H2 d) BaH2 b) H2O e) SO2 c) BaS 12. In the double replacement reaction between the weak acid, HC2H3O2 and strong base, NaOH, which ion(s) are spectator ions? a) Na+, C2H3O2– d) H+, C2 ...
... 11. When H2SO4 and Ba(OH)2 are reacted in a double replacement reaction, one of the products of the reaction is… a) H2 d) BaH2 b) H2O e) SO2 c) BaS 12. In the double replacement reaction between the weak acid, HC2H3O2 and strong base, NaOH, which ion(s) are spectator ions? a) Na+, C2H3O2– d) H+, C2 ...
Final Review
... Balance the following chemical equations using the half-reaction method. SHOW WORK. a. As2O3 (s) + NO3- (aq) H3AsO4 (aq) + NO(g) (in acidic solution) b. CH3OH(aq) + Cr2O72CH2O(aq) + Cr3+ (aq) (in acidic solution) c. CN-(aq) + MnO4 -(aq) CNO-(aq) + MnO2 (s) (in basic solution) d. NO2-(aq) + Al(s) NH3 ...
... Balance the following chemical equations using the half-reaction method. SHOW WORK. a. As2O3 (s) + NO3- (aq) H3AsO4 (aq) + NO(g) (in acidic solution) b. CH3OH(aq) + Cr2O72CH2O(aq) + Cr3+ (aq) (in acidic solution) c. CN-(aq) + MnO4 -(aq) CNO-(aq) + MnO2 (s) (in basic solution) d. NO2-(aq) + Al(s) NH3 ...
Lecture 4 - Winthrop Chemistry, Physics, and Geology
... 1) Geometrical Isomers: The atoms on either side of a bond are arranged differently 2) Optical Isomers: The molecules are each other’s ...
... 1) Geometrical Isomers: The atoms on either side of a bond are arranged differently 2) Optical Isomers: The molecules are each other’s ...
Amines, Amides, & Amino Acids
... bonding capacity of 3.) • Since molecules can contain H bonded to N, hydrogen bonding occurs, but it is weaker than hydrogen bonding in alcohols and carboxylic acids. ...
... bonding capacity of 3.) • Since molecules can contain H bonded to N, hydrogen bonding occurs, but it is weaker than hydrogen bonding in alcohols and carboxylic acids. ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.