4 Organic Chemistry
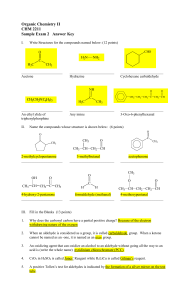
... these two compounds by giving them different names. In this case it is quite straightforward. We call the straight-chain molecule n-butane and the branched molecule iso-butane. However, when alkanes have more than one branch (as many do) we really do need a systematic way of naming them. Rule 1: Cho ...
... these two compounds by giving them different names. In this case it is quite straightforward. We call the straight-chain molecule n-butane and the branched molecule iso-butane. However, when alkanes have more than one branch (as many do) we really do need a systematic way of naming them. Rule 1: Cho ...
Scope and Limitations - Organic Reactions Wiki
... smaller size of the OsO4•quinuclidine complex compared to the OsO4•TMEDA system, increased levels of selectivity are obtained in the directed oxidation of sterically demanding substrates.19 Replacing QNO•H2O, which needs to be prepared beforehand, with commercially available Me3NO•2H2O makes the dih ...
... smaller size of the OsO4•quinuclidine complex compared to the OsO4•TMEDA system, increased levels of selectivity are obtained in the directed oxidation of sterically demanding substrates.19 Replacing QNO•H2O, which needs to be prepared beforehand, with commercially available Me3NO•2H2O makes the dih ...
Organic Chemistry
... Only van der Waals force: London force. Boiling point increases with length of chain. Combust to give mainly CO2 and H2O Nomenclature suffix “‐ane” ...
... Only van der Waals force: London force. Boiling point increases with length of chain. Combust to give mainly CO2 and H2O Nomenclature suffix “‐ane” ...
Organic for Forensic Science
... Benzocaine is often favoured by drug dealers to bulk out their cocaine supplies. while many dealers use the cheaper paracetamol or corn flour, Benzocaine gives a numbing effect (like purer cocaine ...
... Benzocaine is often favoured by drug dealers to bulk out their cocaine supplies. while many dealers use the cheaper paracetamol or corn flour, Benzocaine gives a numbing effect (like purer cocaine ...
fundamentals of structure and reactivity of organic compounds
... 2. Write the rational and IUPAC names of aminalon H2N-CH2-CH2-CH2-COOH. Aminalon participates in brain metabolism. 3. Write the structural formula of 1,1,2-trichloroethane which is used for shortterm narcosis. What class of compounds does it belong to? ...
... 2. Write the rational and IUPAC names of aminalon H2N-CH2-CH2-CH2-COOH. Aminalon participates in brain metabolism. 3. Write the structural formula of 1,1,2-trichloroethane which is used for shortterm narcosis. What class of compounds does it belong to? ...
1 THE BARTON-McCOMBIE REACTION STUART W. McCOMBIE 28
... Professor Derek H. R. Barton, mentor and friend. ...
... Professor Derek H. R. Barton, mentor and friend. ...
13: Carbonyl Compounds: Ketones, Aldehydes, Carboxylic Acids
... less powerful than other Cr(VI) reagents such as Na2 Cr2 O7 , K2 Cr2 O7 , or CrO3 , that are also used to oxidize alcohols. These more powerful reagents not only oxidize 1° alcohols to aldehydes, but further oxidize aldehydes to carboxylic acids. As a result, PCC is a reagent of choice when an aldeh ...
... less powerful than other Cr(VI) reagents such as Na2 Cr2 O7 , K2 Cr2 O7 , or CrO3 , that are also used to oxidize alcohols. These more powerful reagents not only oxidize 1° alcohols to aldehydes, but further oxidize aldehydes to carboxylic acids. As a result, PCC is a reagent of choice when an aldeh ...
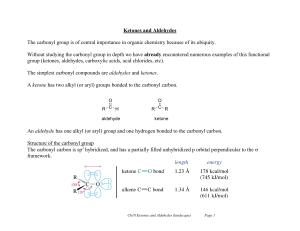
Ketones and Aldehydes
... So much so, that they even attack the lithium salts of carboxylate anions. These dianions can then be protonated, which generates hydrates, which then lose water and produce ketones. E.g. ...
... So much so, that they even attack the lithium salts of carboxylate anions. These dianions can then be protonated, which generates hydrates, which then lose water and produce ketones. E.g. ...
Alkaloids
... These methods are recommended for determination of: 1- Very weak bases which can not be determined by volumetric methods e.g. caffeine and colchicine. 2- Mixtures of alkaloids that are obtained from the same plant but differ greatly in their molecular weight e.g. Cinchona and Rawolfia alkaloids. The ...
... These methods are recommended for determination of: 1- Very weak bases which can not be determined by volumetric methods e.g. caffeine and colchicine. 2- Mixtures of alkaloids that are obtained from the same plant but differ greatly in their molecular weight e.g. Cinchona and Rawolfia alkaloids. The ...
Alkenes 3 - ChemWeb (UCC)
... indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the actual mechanism of the elimination process. Notice that the requirement ...
... indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the actual mechanism of the elimination process. Notice that the requirement ...
Kinetics and Mechanisms of the Reactions of Diaryl
... of the parent species (SiH211,13 and GeH214) with water and alcohols in the gas phase, but there have not yet been any detailed mechanistic studies of the reaction in simple aryl- and alkyl-substituted derivatives by time-resolved kinetic methods in solution at ambient temperatures. ...
... of the parent species (SiH211,13 and GeH214) with water and alcohols in the gas phase, but there have not yet been any detailed mechanistic studies of the reaction in simple aryl- and alkyl-substituted derivatives by time-resolved kinetic methods in solution at ambient temperatures. ...
Lecture - Ch 16
... • On a general basis, there are no reactions between nucleophiles and halobenzenes that do not have electron withdrawing substituents – High temperatures can be used to make chlorobenzene react ...
... • On a general basis, there are no reactions between nucleophiles and halobenzenes that do not have electron withdrawing substituents – High temperatures can be used to make chlorobenzene react ...
laman web smk raja perempuan, ipoh
... (toluene) with Lewis acid catalysts such as AlCl3and FeCl3 and in the presence of light only 22. predict the reaction products when the substitution group in benzene is an electron accepting or donating group ...
... (toluene) with Lewis acid catalysts such as AlCl3and FeCl3 and in the presence of light only 22. predict the reaction products when the substitution group in benzene is an electron accepting or donating group ...
Chem E2b - Organic Chemistry II What is Organic Chemistry?
... • Halogens are deactivating (inductive effect) and ortho/para directing (resonance) • Nucleophiles can add to arenediazonium salts, strongly activated aryl halides (SNAr), or benzyne intermediates • 5-Membered heteroaromatics participate in EAS at C2position. • 6-Membered heteroaromatics participate ...
... • Halogens are deactivating (inductive effect) and ortho/para directing (resonance) • Nucleophiles can add to arenediazonium salts, strongly activated aryl halides (SNAr), or benzyne intermediates • 5-Membered heteroaromatics participate in EAS at C2position. • 6-Membered heteroaromatics participate ...
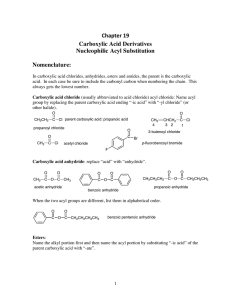
Chapter 19 Carboxylic Acid Derivatives Nucleophilic Acyl
... For thioesters: Sulfur is a third row element, like chlorine, and so it is considerably larger than oxygen. The C-S bond is relatively long and this makes for poor overlap of the lone pair 3p orbital and the π-orbital of the carbonyl. But thioesters are less reactive than acid chlorides and anhydrid ...
... For thioesters: Sulfur is a third row element, like chlorine, and so it is considerably larger than oxygen. The C-S bond is relatively long and this makes for poor overlap of the lone pair 3p orbital and the π-orbital of the carbonyl. But thioesters are less reactive than acid chlorides and anhydrid ...
Title Several Reactions of Isocyanide and Related Compounds
... mechanism through a mixed ligand complex has been proposed, in which both isocyanide and amine are coordinated with the common metal ion and the reaction takes place in the sphere of complex ligand. In Chapter 2, insertion reactions of isocyanide between oxygen and hydrogen bond of alcohols to produ ...
... mechanism through a mixed ligand complex has been proposed, in which both isocyanide and amine are coordinated with the common metal ion and the reaction takes place in the sphere of complex ligand. In Chapter 2, insertion reactions of isocyanide between oxygen and hydrogen bond of alcohols to produ ...
Lecture - Ch 17
... with the use of a strong acid as catalyst • Reactivity of carboxylic acid is increased by converting it into a carboxylic acid chloride, which then reacts with the alcohol ...
... with the use of a strong acid as catalyst • Reactivity of carboxylic acid is increased by converting it into a carboxylic acid chloride, which then reacts with the alcohol ...
Ch 16 Amines
... • Basicity and Blood – Most Aliphatic amines are fairly strong bases – How will they be found in the blood stream? ...
... • Basicity and Blood – Most Aliphatic amines are fairly strong bases – How will they be found in the blood stream? ...
Graphene-Catalyzed Direct Friedel–Crafts Alkylation Reactions
... produce valuable diarylalkane products21 in high yields and excellent regioselectivity. This process constitutes the first general application of graphenes to promote direct C−C bond formation, utilizing polar functional groups anchored on the GO surface,23−30 which may open the door for industrial a ...
... produce valuable diarylalkane products21 in high yields and excellent regioselectivity. This process constitutes the first general application of graphenes to promote direct C−C bond formation, utilizing polar functional groups anchored on the GO surface,23−30 which may open the door for industrial a ...
Isomeric Product Detection in the
... branched alkene with six CC double bonds) and linolenic acid (C18H30O2, a linear carboxylic acid with three CC double bonds) with OH radicals are identified and quantified using two-dimensional gas chromatography−mass spectrometry. The reactions are measured at low and high [O2] (∼1% vs 10% [O2]) to ...
... branched alkene with six CC double bonds) and linolenic acid (C18H30O2, a linear carboxylic acid with three CC double bonds) with OH radicals are identified and quantified using two-dimensional gas chromatography−mass spectrometry. The reactions are measured at low and high [O2] (∼1% vs 10% [O2]) to ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.