Name_____________________________________ 22 • Organic
... is added. The resulting product will react with a base to form a salt and water. When the new product in container X is mixed with container Y and H2SO4 is added, the chemist is able to confirm that: ...
... is added. The resulting product will react with a base to form a salt and water. When the new product in container X is mixed with container Y and H2SO4 is added, the chemist is able to confirm that: ...
Reactions of Alcohols
... • Methanol oxidizes to formaldehyde, then formic acid, more toxic than methanol. • Ethylene glycol oxidizes to oxalic acid, toxic. • Treatment for poisoning is excess ethanol. ...
... • Methanol oxidizes to formaldehyde, then formic acid, more toxic than methanol. • Ethylene glycol oxidizes to oxalic acid, toxic. • Treatment for poisoning is excess ethanol. ...
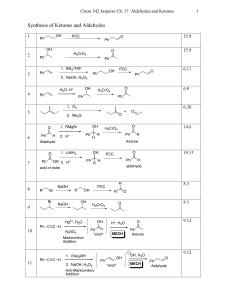
Synthesis of Ketones and Aldehydes
... Classification of Mechanisms Associated With Ketone/Aldehyde Reactions. • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict wh ...
... Classification of Mechanisms Associated With Ketone/Aldehyde Reactions. • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict wh ...
Organic_1_6.1ed_2012_02nd_module_functional_groups_and_IR
... C=O: 1630-1780 cm-1, strong absorption If you need to use other frequencies to identify other functional groups (and sometimes you will), a table of IR frequencies will be provided. ...
... C=O: 1630-1780 cm-1, strong absorption If you need to use other frequencies to identify other functional groups (and sometimes you will), a table of IR frequencies will be provided. ...
Chapter 11 Reactions of Alcohols Types of Alcohol Reactions
... • New O-C bond forms, O-H bond breaks. ...
... • New O-C bond forms, O-H bond breaks. ...
Oxidation
... Reaction rate is insensitive to solvent polarity implying concerted mechanism without intermediacy of ionic intermediate Less hindered face of olefin is epoxide ...
... Reaction rate is insensitive to solvent polarity implying concerted mechanism without intermediacy of ionic intermediate Less hindered face of olefin is epoxide ...
The story of V
... do not have left reactive groups in the matrix: no reactive end groups (-OH, -COOH), no unreacted carboncarbon double bonds (= unsaturated, (vinyl group = ethenyl group = unsaturated end-group)). Vinyl-Ester (VE) systems, like most resin systems, shrink during the curing (polymerisation). The volume ...
... do not have left reactive groups in the matrix: no reactive end groups (-OH, -COOH), no unreacted carboncarbon double bonds (= unsaturated, (vinyl group = ethenyl group = unsaturated end-group)). Vinyl-Ester (VE) systems, like most resin systems, shrink during the curing (polymerisation). The volume ...
Alkenes notes
... thus attract electrophiles and undergo heterolytic fission. Heterolytic fission is the breaking of a covalent bond which results in both electrons going to the same atom. This is in contrast to alkanes which can only react with free radicals and undergo homolytic fission. An electrophile is a specie ...
... thus attract electrophiles and undergo heterolytic fission. Heterolytic fission is the breaking of a covalent bond which results in both electrons going to the same atom. This is in contrast to alkanes which can only react with free radicals and undergo homolytic fission. An electrophile is a specie ...
Organic Chemistry Fifth Edition
... Dimethylallyl diphosphate has a leaving group (diphosphate) at an allylic carbon; it is reactive toward nucleophilic substitution at this position. ...
... Dimethylallyl diphosphate has a leaving group (diphosphate) at an allylic carbon; it is reactive toward nucleophilic substitution at this position. ...
Urea-formaldehyde (UF) resins have been one of the mainstays of
... modification is selected from the group consisting of (1) esterification of said maleated unsaturated fatty acids with ricinoleic acid, (2) amidation of said maleated unsaturated fatty acids using a polyamine supplied in an amount sufficient to cause cross linking between maleated fatty acid molecul ...
... modification is selected from the group consisting of (1) esterification of said maleated unsaturated fatty acids with ricinoleic acid, (2) amidation of said maleated unsaturated fatty acids using a polyamine supplied in an amount sufficient to cause cross linking between maleated fatty acid molecul ...
06. Alcohols. Phenols. Ethers
... the dialkyloxonium ion. This step gives one molecule of an alkyl halide and one molecule of an alcohol. ...
... the dialkyloxonium ion. This step gives one molecule of an alkyl halide and one molecule of an alcohol. ...
RUMPLE-DISSERTATION-2014 - SMARTech Home
... Eckert. The opportunity to work with such skilled scientists and kind mentors is a rare one, and I am extremely glad I had the opportunity to learn from them. I have always been in awe of their brilliance (I’m pretty sure they have each forgotten more than I’ll ever learn, and they still know so muc ...
... Eckert. The opportunity to work with such skilled scientists and kind mentors is a rare one, and I am extremely glad I had the opportunity to learn from them. I have always been in awe of their brilliance (I’m pretty sure they have each forgotten more than I’ll ever learn, and they still know so muc ...
homogeneous catalysis
... and industrial research laboratories the growth in research activity in this area in the past decade or so has been phenomenal. Written mainly from a pedagogical point of view, this book is not comprehensive but selective. The material presented was selected on the basis of two criteria. We have tri ...
... and industrial research laboratories the growth in research activity in this area in the past decade or so has been phenomenal. Written mainly from a pedagogical point of view, this book is not comprehensive but selective. The material presented was selected on the basis of two criteria. We have tri ...
Thermodynamics and kinetics of the hydrolysis of atmospherically
... For the separately prepared organonitrates (ethyl nitrate, isopropyl nitrate, 1-nitrato-2-butanol, 2-nitrato-1-butanol, 3nitrato-2-butanol, and 2-methyl-2-nitrato-1-butanol), the reaction mixtures were prepared and subjected to NMR analysis at room temperature (296 ± 2 K) as follows. A 5.0 ml aliquo ...
... For the separately prepared organonitrates (ethyl nitrate, isopropyl nitrate, 1-nitrato-2-butanol, 2-nitrato-1-butanol, 3nitrato-2-butanol, and 2-methyl-2-nitrato-1-butanol), the reaction mixtures were prepared and subjected to NMR analysis at room temperature (296 ± 2 K) as follows. A 5.0 ml aliquo ...
Hydrogenation for Low Trans and High Conjugated Fatty Acids
... current density. Current pulsing for frequencies in the range of 0.25 to 60 Hz had no effect on current efficiencies. The combination of increased oil feed flow rate and inserted nickel turbulence promoter into the oil stream increased the current efficiency of oil hydrogenation. An and others (1999 ...
... current density. Current pulsing for frequencies in the range of 0.25 to 60 Hz had no effect on current efficiencies. The combination of increased oil feed flow rate and inserted nickel turbulence promoter into the oil stream increased the current efficiency of oil hydrogenation. An and others (1999 ...
Chiral Enolate Equivalents
... confines the range of usable electrophiles to aldehydes, some primary or activated alkyl halides, unsaturated carbonyls, electrophilic halogens, oxaziridines, aza compounds, and a handful of other reactive electrophiles.3 Intramolecular reactions may tolerate slightly less reactive electrophiles. Wi ...
... confines the range of usable electrophiles to aldehydes, some primary or activated alkyl halides, unsaturated carbonyls, electrophilic halogens, oxaziridines, aza compounds, and a handful of other reactive electrophiles.3 Intramolecular reactions may tolerate slightly less reactive electrophiles. Wi ...
CHEM 203 Material
... reactivity: Example: the C atom in CH4 has formally acquired 4 electrons, thereby assuming the oxidation state of –4. This produces a significant concentration of electronic density around the C atom. One may predict that the C atom in methane will behave as an electron donor in its reactions; that ...
... reactivity: Example: the C atom in CH4 has formally acquired 4 electrons, thereby assuming the oxidation state of –4. This produces a significant concentration of electronic density around the C atom. One may predict that the C atom in methane will behave as an electron donor in its reactions; that ...
APPROACHES TO CARBOHYDRATE-BASED CHEMICAL LIBRARIES: THE
... finishing (work-up and purification) them. In order for parallel synthesis to meet its goal of drastically reducing the time per chemist per compound, the rate-limiting step in the ...
... finishing (work-up and purification) them. In order for parallel synthesis to meet its goal of drastically reducing the time per chemist per compound, the rate-limiting step in the ...
The Impact of Amino Acids on Growth Performance
... Unfortunately, these studies concerning the impact of amino acids on the production of aroma compounds are often under conditions which are not reproducible (real grape must) or utilizing very complex nitrogen treatments. Due to these factors, it is problematic to interpret the results from a metabo ...
... Unfortunately, these studies concerning the impact of amino acids on the production of aroma compounds are often under conditions which are not reproducible (real grape must) or utilizing very complex nitrogen treatments. Due to these factors, it is problematic to interpret the results from a metabo ...
Postprint
... With N,P-ligated iridium catalysts, the enantio-selectivity stems from the bulk of the chiral ligand. A quadrant model was devised to rationalise and also predict the configuration of the resulting product. As shown in Figure 3, the iridium centre is surrounded by two bulky groups that generate ster ...
... With N,P-ligated iridium catalysts, the enantio-selectivity stems from the bulk of the chiral ligand. A quadrant model was devised to rationalise and also predict the configuration of the resulting product. As shown in Figure 3, the iridium centre is surrounded by two bulky groups that generate ster ...
Aromatic Compounds
... • Addition of a reagent such as HCl to an alkene • The electrophilic hydrogen approaches the p electrons ...
... • Addition of a reagent such as HCl to an alkene • The electrophilic hydrogen approaches the p electrons ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.