Aromatic Compounds
... • Addition of a reagent such as HCl to an alkene • The electrophilic hydrogen approaches the p electrons ...
... • Addition of a reagent such as HCl to an alkene • The electrophilic hydrogen approaches the p electrons ...
Chapter - FIU Faculty Websites
... equilibrium can be driven to the right by removing H2O as it is formed using distillation or other techniques. Please note that hemiacetals can be hydrolyzed back to aldehydes or ketones in acidic and basic solutions while acetals can be only hydrolyzed to carbonyl compounds in acidic conditions and ...
... equilibrium can be driven to the right by removing H2O as it is formed using distillation or other techniques. Please note that hemiacetals can be hydrolyzed back to aldehydes or ketones in acidic and basic solutions while acetals can be only hydrolyzed to carbonyl compounds in acidic conditions and ...
Ans:- (i) Gluconic acid - Kendriya Vidyalaya No.2, Kribhco, Surat
... Q- 18. What is the basic principle of fuel cell ? give two advantages of fuel cell. Ans. FUEL CELL is used to convert the chemical energy of fuel into electrical energy Chemical reactions: Reaction at O2 ( g ) 2 H 2O(l ) 4e 4OH (aq) Cathode 2 H 2 4OH 4 H 2O 4e Anode 2H 2 ( g ) ...
... Q- 18. What is the basic principle of fuel cell ? give two advantages of fuel cell. Ans. FUEL CELL is used to convert the chemical energy of fuel into electrical energy Chemical reactions: Reaction at O2 ( g ) 2 H 2O(l ) 4e 4OH (aq) Cathode 2 H 2 4OH 4 H 2O 4e Anode 2H 2 ( g ) ...
Ch 7 - Practice problem (Answers)
... Chem 341: Organic Chemistry, Prof. Jayaraman Sivaguru, Fall 2016. ...
... Chem 341: Organic Chemistry, Prof. Jayaraman Sivaguru, Fall 2016. ...
research reviews Discovering new arene-catalyzed lithiations
... new organolithium intermediate having at least two more carbon atoms [50]. A significant advantage of this process is that the new organolithium can then react with an electrophile, such that in only one synthetic operation profound changes can take place in the starting material. Carbolithiation ca ...
... new organolithium intermediate having at least two more carbon atoms [50]. A significant advantage of this process is that the new organolithium can then react with an electrophile, such that in only one synthetic operation profound changes can take place in the starting material. Carbolithiation ca ...
Synthesis of Natural Products and Related Compounds using Enyne
... our group (Scheme 6).[22] Reactions of five- to sevenmembered cycloalkenes 14 having the substituent at the 3-position of the cycloalkene with 1c under ethylene gas afforded the cyclic compounds 15 in good yields. This reaction could proceed via the highly strained ruthenacyclobutane 16. In each cas ...
... our group (Scheme 6).[22] Reactions of five- to sevenmembered cycloalkenes 14 having the substituent at the 3-position of the cycloalkene with 1c under ethylene gas afforded the cyclic compounds 15 in good yields. This reaction could proceed via the highly strained ruthenacyclobutane 16. In each cas ...
Iodomethylzinc_iodid.. - Groupe Charette
... the Zn–Cu couple with CH2 I2 and a crystal of Iodine in Et2 O followed by heating to reflux generates the active reagent. Other modifications include the use of CH2 I2 /Zn/CuCl,12a CH2 I2 /Zn–Ag couple,12b CH2 Br2 /Zn/TiCl4 ,12c and CH2 Br2 /Zn/AcCl/CuCl.12d Type 2 reagent generation has been utiliz ...
... the Zn–Cu couple with CH2 I2 and a crystal of Iodine in Et2 O followed by heating to reflux generates the active reagent. Other modifications include the use of CH2 I2 /Zn/CuCl,12a CH2 I2 /Zn–Ag couple,12b CH2 Br2 /Zn/TiCl4 ,12c and CH2 Br2 /Zn/AcCl/CuCl.12d Type 2 reagent generation has been utiliz ...
50 chemistry questions for class xii
... For example- heart has more β adrenergic receptors than α adrenergic receptors .This means that a drug designed to interact with β adrenergic receptors will act on heart rather than a tissue which are rich in α adrenergic receptors . Q49 Define the following terms with one example(Any two) (a) Antip ...
... For example- heart has more β adrenergic receptors than α adrenergic receptors .This means that a drug designed to interact with β adrenergic receptors will act on heart rather than a tissue which are rich in α adrenergic receptors . Q49 Define the following terms with one example(Any two) (a) Antip ...
Reactions You Should Know When You Begin Organic II
... Adding agent can be symmetrical or asymmetrical. Ex. H2 vs. HCl Symmetrical: H2, C12, Br2, and I2 (I2 slow and readily reversible) Asymmetrical: HCl, RBr, HOH (H2O) Addition of symmetrical agents may be anti or syn depending on mechanism or catalyst. Addition of asymmetrical agents follows Markovnik ...
... Adding agent can be symmetrical or asymmetrical. Ex. H2 vs. HCl Symmetrical: H2, C12, Br2, and I2 (I2 slow and readily reversible) Asymmetrical: HCl, RBr, HOH (H2O) Addition of symmetrical agents may be anti or syn depending on mechanism or catalyst. Addition of asymmetrical agents follows Markovnik ...
phenol
... 7. Chemical properties of monohydroxyl alcohols Alcohols are classified as primary (1'), secondary (2'), or tertiary (3'), depending on the number of carbon atoms bonded to the carbon atom that bears the hydroxyl group. А primary alcohol is an alcohol in which the hydroxyl-bearing carbon atom is at ...
... 7. Chemical properties of monohydroxyl alcohols Alcohols are classified as primary (1'), secondary (2'), or tertiary (3'), depending on the number of carbon atoms bonded to the carbon atom that bears the hydroxyl group. А primary alcohol is an alcohol in which the hydroxyl-bearing carbon atom is at ...
1.4. Nomenclature 2. LABORATORY
... 11was latcr found by Moser and Lindinger3 that the addition of small quantities ' certain metal salts, particularly copper salts, to the reaction mixture accelerated reduction of ethylene, whereas the addition of mercuric oxide and molybdic oxide rrmoted the formation of sulphur dioxide. By using a ...
... 11was latcr found by Moser and Lindinger3 that the addition of small quantities ' certain metal salts, particularly copper salts, to the reaction mixture accelerated reduction of ethylene, whereas the addition of mercuric oxide and molybdic oxide rrmoted the formation of sulphur dioxide. By using a ...
Organic Chemistry II
... Alkyl Halides We did talk about halo-alkanes (called alkyl halides) which are alkanes with a halogen attached. These molecules do, in fact, have polar bonds: C-Br, C-I, C-Cl are all polar bonds. Carbon is slightly positive, the halogen is slightly negative. ...
... Alkyl Halides We did talk about halo-alkanes (called alkyl halides) which are alkanes with a halogen attached. These molecules do, in fact, have polar bonds: C-Br, C-I, C-Cl are all polar bonds. Carbon is slightly positive, the halogen is slightly negative. ...
Improved Synthesis, Separation, Transition Metal Coordination and
... The 6-10.5 ppm region of the 1H NMR spectra: sample from reaction with 1-hexene, meso-(et,ph-P4) in acetone-d6/ D2O under N2, 24 hours (blue spectrum), same as above recorded 1.5 hours after exposure to O2(red spectrum).......................................................... 68 ...
... The 6-10.5 ppm region of the 1H NMR spectra: sample from reaction with 1-hexene, meso-(et,ph-P4) in acetone-d6/ D2O under N2, 24 hours (blue spectrum), same as above recorded 1.5 hours after exposure to O2(red spectrum).......................................................... 68 ...
Chapter Seven PPT
... • Hydrocarbons Containing Double and Triple Bonds • Unsaturated Compounds (Less than Maximum H Atoms) • Alkenes also Referred to as Olefins • Properties Similar to those of Corresponding Alkanes • Slightly Soluble in Water • Dissolve Readily in Nonpolar or Low Polarity Solvents • Densities of Alkene ...
... • Hydrocarbons Containing Double and Triple Bonds • Unsaturated Compounds (Less than Maximum H Atoms) • Alkenes also Referred to as Olefins • Properties Similar to those of Corresponding Alkanes • Slightly Soluble in Water • Dissolve Readily in Nonpolar or Low Polarity Solvents • Densities of Alkene ...
Lithium Bromide Original Commentary
... Weak Lewis Acid. Lithium bromide is used as a mild Lewis acid in a variety of reactions. For example, this reagent was used in the Pictet-Spengler cyclization of a highly functionalized imine (eq 13).33 In this reaction, carbon-carbon bond formation occurs without reaction or loss of stereochemical ...
... Weak Lewis Acid. Lithium bromide is used as a mild Lewis acid in a variety of reactions. For example, this reagent was used in the Pictet-Spengler cyclization of a highly functionalized imine (eq 13).33 In this reaction, carbon-carbon bond formation occurs without reaction or loss of stereochemical ...
How many possible trisubstituted derivatives C6H3X2Y can be
... How many possible trisubstituted derivatives C6H3X2Y can be btained from the meta disubstituted benzene C6H4X2? (Ignore directive effects of the substituents X and assume that all positions on the ring are accessible to the reagent Y+). 1. One 2. Two 3. Three 4. four ...
... How many possible trisubstituted derivatives C6H3X2Y can be btained from the meta disubstituted benzene C6H4X2? (Ignore directive effects of the substituents X and assume that all positions on the ring are accessible to the reagent Y+). 1. One 2. Two 3. Three 4. four ...
10 Haloalkanes and Haloarenes
... substitution reaction product instead of addition reaction product. This is because at higher temperature, the addition reaction is reversible and the substitution reaction is irreversible. The hydrogen atom of allylic carbon is replaced with the halogen atom to form allylic halides and the reaction ...
... substitution reaction product instead of addition reaction product. This is because at higher temperature, the addition reaction is reversible and the substitution reaction is irreversible. The hydrogen atom of allylic carbon is replaced with the halogen atom to form allylic halides and the reaction ...
haloalkanes and arenes
... held together by dipole-dipole interactions. Similarly, strong H-bonds exist between the water molecules. The new force of attraction between the alkyl halides and water molecules is weaker than the alkyl halide-alkyl halide and water-water forces of attraction. Hence, alkyl halides (though polar) a ...
... held together by dipole-dipole interactions. Similarly, strong H-bonds exist between the water molecules. The new force of attraction between the alkyl halides and water molecules is weaker than the alkyl halide-alkyl halide and water-water forces of attraction. Hence, alkyl halides (though polar) a ...
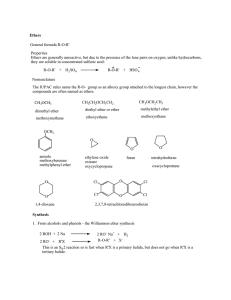
Ethers General formula R-O-R` Properties Ethers are generally
... CH3 CH3 Hg(OCOCH 3)2/CH3 CH2OH H2C C CH 3 H2C C CH 3 Hg OCH2 CH3 O C CH 3 ...
... CH3 CH3 Hg(OCOCH 3)2/CH3 CH2OH H2C C CH 3 H2C C CH 3 Hg OCH2 CH3 O C CH 3 ...
SOLID STATE KEY CONCEPTS As we know that matter exists in different physical states under different conditions
... Solution is the homogeneous mixture of two or more substances in which the components are uniformly distributed into each other. The substances which make the solution are called components. Most of the solutions are binary i.e., consists of two components out of which one is solute and other is s ...
... Solution is the homogeneous mixture of two or more substances in which the components are uniformly distributed into each other. The substances which make the solution are called components. Most of the solutions are binary i.e., consists of two components out of which one is solute and other is s ...
Palladium(II)-Catalyzed Oxidative Cyclization Strategies Andreas K. Å. Persson
... The use of transition metal catalysts has proven to be one of the most diverse tools for the mild and selective formation of carbon-carbon bonds. In particular palladium-catalyzed cross-coupling reactions have revolutionized the field. The main focus of this thesis has been directed towards preparat ...
... The use of transition metal catalysts has proven to be one of the most diverse tools for the mild and selective formation of carbon-carbon bonds. In particular palladium-catalyzed cross-coupling reactions have revolutionized the field. The main focus of this thesis has been directed towards preparat ...
14_06_10.html
... Synthesis of Alcohols Using Organolithium Reagents Organolithium reagents react with aldehydes and ketones in the same way that Grignard reagents do. ...
... Synthesis of Alcohols Using Organolithium Reagents Organolithium reagents react with aldehydes and ketones in the same way that Grignard reagents do. ...
Polymerization Synthesis of Nylon 6,10 C11-5
... Making nylon is even easier if you use a diamine and a diacid chloride instead of a diacid. This is because acid chlorides are much more reactive than acids. The reaction is done in a two-phase system. The amine is dissolved in water, and the diacid chloride in an organic solvent. The two solutions ...
... Making nylon is even easier if you use a diamine and a diacid chloride instead of a diacid. This is because acid chlorides are much more reactive than acids. The reaction is done in a two-phase system. The amine is dissolved in water, and the diacid chloride in an organic solvent. The two solutions ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.