Class Notes Test 1
... Super Strong Bases and Nucleophiles • The counterion metal is a spectator • Stability-reactivity principle: very unstable à very reactive • This great reactivity is very useful (as nucleophile) • This great reactivity (as base) has implication for proper technical use (see following) 7. Solvent and ...
... Super Strong Bases and Nucleophiles • The counterion metal is a spectator • Stability-reactivity principle: very unstable à very reactive • This great reactivity is very useful (as nucleophile) • This great reactivity (as base) has implication for proper technical use (see following) 7. Solvent and ...
Oxidation involving CO System ( O
... cannot be further converted to the acid. The aldehyde is converted back to an alcohol by alcohol (keto) reductases (reversible), however, it goes forward as the aldehyde is converted to carboxylic acid; 3° alcohols and phenolic alcohols cannot be oxidized by this enzyme; No “H” attached to adjacent ...
... cannot be further converted to the acid. The aldehyde is converted back to an alcohol by alcohol (keto) reductases (reversible), however, it goes forward as the aldehyde is converted to carboxylic acid; 3° alcohols and phenolic alcohols cannot be oxidized by this enzyme; No “H” attached to adjacent ...
Chapter 22: Phenols. Alcohols contain an OH group bonded to an
... OH group of phenols can participate in hydrogen bonding with other phenol molecules and to water. 22.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
... OH group of phenols can participate in hydrogen bonding with other phenol molecules and to water. 22.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
Chapter 7 Hydrosilylation of Carbon
... to the participation of π-benzylic palladium intermediates [1, 2]. It is known that bisphosphine-palladium complexes are catalytically much less active than monophosphine-palladium complexes and hence asymmetric synthesis has been attempted by use of chiral monodentate phosphine ligands. In the first ...
... to the participation of π-benzylic palladium intermediates [1, 2]. It is known that bisphosphine-palladium complexes are catalytically much less active than monophosphine-palladium complexes and hence asymmetric synthesis has been attempted by use of chiral monodentate phosphine ligands. In the first ...
CH 2
... that contains one or more carbon – carbon double bonds. ALKYNES – An unsaturated hydrocarbon that contains one or more carbon – carbon triple bonds. AROMATIC – Organic compounds that contains the characteristics of benzene & benzene ring in its structure. ...
... that contains one or more carbon – carbon double bonds. ALKYNES – An unsaturated hydrocarbon that contains one or more carbon – carbon triple bonds. AROMATIC – Organic compounds that contains the characteristics of benzene & benzene ring in its structure. ...
Document
... 29 NAD+/NADH • NAD+ is involved in a variety of enzyme-catalyzed oxidation/reduction reactions; we deal with two of these in this chapter ...
... 29 NAD+/NADH • NAD+ is involved in a variety of enzyme-catalyzed oxidation/reduction reactions; we deal with two of these in this chapter ...
Anionic rearrangement of 2-benzyloxypyridine derivatives and a synthetic approach to aldingenin B
... [1,2]-Anionic rearrangements are important tools for altering the complexity of molecules at hand. In Part I of this dissertation, an anionic rearrangement of 2-benzyloxypyridine is described. Pyridine-directed metallation of the benzylic carbon leads to 1,2-migration of pyridine via a postulated as ...
... [1,2]-Anionic rearrangements are important tools for altering the complexity of molecules at hand. In Part I of this dissertation, an anionic rearrangement of 2-benzyloxypyridine is described. Pyridine-directed metallation of the benzylic carbon leads to 1,2-migration of pyridine via a postulated as ...
13: Carbonyl Compounds: Ketones, Aldehydes, Carboxylic Acids
... Other Cr(VI) Reagents. PCC is a specialized oxidizing reagent that is less powerful than other Cr(VI) reagents such as Na2Cr2O7, K2Cr2O7, or CrO3, that are also used to oxidize alcohols. These more powerful reagents not only oxidize 1° alcohols to aldehydes, but further oxidize aldehydes to carboxyl ...
... Other Cr(VI) Reagents. PCC is a specialized oxidizing reagent that is less powerful than other Cr(VI) reagents such as Na2Cr2O7, K2Cr2O7, or CrO3, that are also used to oxidize alcohols. These more powerful reagents not only oxidize 1° alcohols to aldehydes, but further oxidize aldehydes to carboxyl ...
Neuman Chapter - Department of Chemistry
... The leaving group L (Figure 9.02) is a halogen X. Because we refer to the C-X carbon as Cα, and its adjacent C-H carbon as Cβ, we say that the H on Cβ is a β-hydrogen or a β-H. The elimination reactions of haloalkanes illustrate the fundamental features and mechanisms of many elimination reactions t ...
... The leaving group L (Figure 9.02) is a halogen X. Because we refer to the C-X carbon as Cα, and its adjacent C-H carbon as Cβ, we say that the H on Cβ is a β-hydrogen or a β-H. The elimination reactions of haloalkanes illustrate the fundamental features and mechanisms of many elimination reactions t ...
Now! - Soojeede.com
... an accomplishment for a scientist in the late 19 century with very little technology but with the combination of knowledge and intellect available at the time. Arrhenius led the way to our understanding of how acids and bases differed, their properties, and their reactions. We may not realize how mu ...
... an accomplishment for a scientist in the late 19 century with very little technology but with the combination of knowledge and intellect available at the time. Arrhenius led the way to our understanding of how acids and bases differed, their properties, and their reactions. We may not realize how mu ...
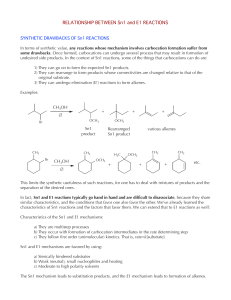
RELATIONSHIP BETWEEN Sn1 and E1 REACTIONS
... This limits the synthetic usefulness of such reactions, for one has to deal with mixtures of products and the separation of the desired ones. In fact, Sn1 and E1 reactions typically go hand in hand and are difficult to disassociate, because they share similar characteristics, and the conditions that ...
... This limits the synthetic usefulness of such reactions, for one has to deal with mixtures of products and the separation of the desired ones. In fact, Sn1 and E1 reactions typically go hand in hand and are difficult to disassociate, because they share similar characteristics, and the conditions that ...
Organic Chemistry - Functional Groups
... D. hex-6-al-3-ene E. hexene6-3-al Principles of Chemistry II ...
... D. hex-6-al-3-ene E. hexene6-3-al Principles of Chemistry II ...
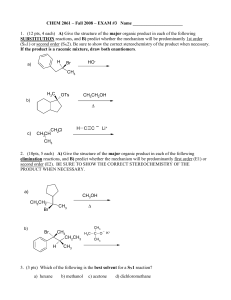
Exam #3
... (12 pts) Bromoetherification, the addition of the elements Br and OR to a double bond, is a common method for constructing rings containing oxygen atoms. Draw a stepwise mechanism for the following INTRAMOLECULAR bromoetherification reaction. Hint: the mechanism is analogous to that of bromohydrin f ...
... (12 pts) Bromoetherification, the addition of the elements Br and OR to a double bond, is a common method for constructing rings containing oxygen atoms. Draw a stepwise mechanism for the following INTRAMOLECULAR bromoetherification reaction. Hint: the mechanism is analogous to that of bromohydrin f ...
Full Text
... Protection of functional groups in multistep organic syntheses is one of the key factors in the success of the synthesis. The protecting group should selectively react in good yield to give a protected substrate and should be selectively removed in good yield by readily available, preferably nontoxi ...
... Protection of functional groups in multistep organic syntheses is one of the key factors in the success of the synthesis. The protecting group should selectively react in good yield to give a protected substrate and should be selectively removed in good yield by readily available, preferably nontoxi ...
A Practical RuCl3-Catalyzed Oxidation Using Trichloroisocyanuric
... reaction (Scheme 1). However, besides the hazardous nature of the chromium species in PDC, use of the polar solvent of DMF has kept the PDC-mediated oxidation from being practical due to the difficulty in separating the carboxylic acid products 3 from DMF through the extractive workup.9 To effect th ...
... reaction (Scheme 1). However, besides the hazardous nature of the chromium species in PDC, use of the polar solvent of DMF has kept the PDC-mediated oxidation from being practical due to the difficulty in separating the carboxylic acid products 3 from DMF through the extractive workup.9 To effect th ...
ALCOHOLS, PHENOLS AND ETHERS
... According to the IUPAC system of nomenclature, alcohols are called alkanols. They are named as the derivatives of the corresponding alkane in which the -e of the alkane is replaced by -ol . The procedure for nomenclature involves the following steps: Step 1: Select the longest carbon chain which con ...
... According to the IUPAC system of nomenclature, alcohols are called alkanols. They are named as the derivatives of the corresponding alkane in which the -e of the alkane is replaced by -ol . The procedure for nomenclature involves the following steps: Step 1: Select the longest carbon chain which con ...
JOURNAL OF FLOW CHEMISTRY (ISSN: 2062
... observed with most of the biocatalysts (Table 1, Panel A). Enantiomeric excess of the product (R,R)-3a was predominantly at least 99.9%. Moreover, this could be realised at conversions of 35% or above with five lipase preparations while KR was almost complete (nearly 50% conversion) with CaLB N435 o ...
... observed with most of the biocatalysts (Table 1, Panel A). Enantiomeric excess of the product (R,R)-3a was predominantly at least 99.9%. Moreover, this could be realised at conversions of 35% or above with five lipase preparations while KR was almost complete (nearly 50% conversion) with CaLB N435 o ...
Recent advances in homogeneous nickel catalysis
... have been disclosed. Benzylic cross-coupling Another mode of bond activation that has come to prominence, at least in part thanks to nickel catalysis, is the activation of benzylic C–O bonds37. Benzylic ethers, esters, carbonates, carbamates and, in some instances, even free alcohols (via the corre ...
... have been disclosed. Benzylic cross-coupling Another mode of bond activation that has come to prominence, at least in part thanks to nickel catalysis, is the activation of benzylic C–O bonds37. Benzylic ethers, esters, carbonates, carbamates and, in some instances, even free alcohols (via the corre ...
Enzymatic synthesis of bioactive compounds
... concentrations and seem to prevent enzyme inactivation because they show an acid-suppressive effect due to their basicity (Hasegawa et al. 2008). However, esterification of glycolic acid has been favored in apolar hexane producing high yield of glycolate ester (91 % after 24 h) (Torres and Otero 199 ...
... concentrations and seem to prevent enzyme inactivation because they show an acid-suppressive effect due to their basicity (Hasegawa et al. 2008). However, esterification of glycolic acid has been favored in apolar hexane producing high yield of glycolate ester (91 % after 24 h) (Torres and Otero 199 ...
Oxidative Alihatic Carbon-Carbon Bond Cleavage Reactions
... biomass for fuel production, applications in wastewater treatment and bioremediation, and in developing new reactions for organic synthesis of fine chemicals including pharmaceuticals. Ideally these reactions would be carried out with high atom economy at low temperatures and pressures, and using ea ...
... biomass for fuel production, applications in wastewater treatment and bioremediation, and in developing new reactions for organic synthesis of fine chemicals including pharmaceuticals. Ideally these reactions would be carried out with high atom economy at low temperatures and pressures, and using ea ...
PHYSICOCHEMICAL PROPERTIES OF ORGANIC MEDICINAL
... As in the case of the alcohols, the -OH function of phenols provides a dipole that significantly affects the physicochemical properties. The aromatic portion of phenols is relatively non-polar (lipophilic), thus phenols exhibit limited water solubility. Also, addition of lipophilic substituents to t ...
... As in the case of the alcohols, the -OH function of phenols provides a dipole that significantly affects the physicochemical properties. The aromatic portion of phenols is relatively non-polar (lipophilic), thus phenols exhibit limited water solubility. Also, addition of lipophilic substituents to t ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.