* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ch 16 Amines
Survey
Document related concepts
Transcript
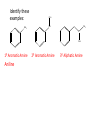
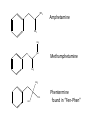
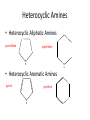
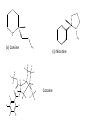
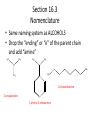
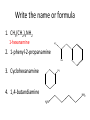
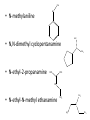
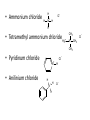
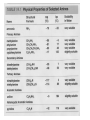
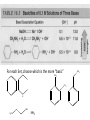
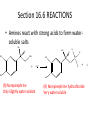
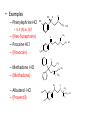
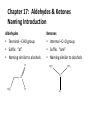
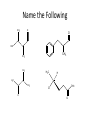
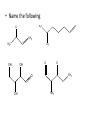
Ch 16 Amines Organic and Biochemsity Section 16.1 Introduction • Amines – Nitrogen found in amine group is the fourth most common element in organic chemistry – Known for their basicity Section 16.2 Classification and Structure • Primary 1⁰ R NH2 • Secondary R 2⁰ R • Tertiary NH R 3⁰ R N R • Aliphatic Amines – All carbon groups bonded to the nitrogen are ALKYL groups • Aromatic Amines – One or more groups bonded to the nitrogen are ARYL groups • Heterocyclic Amines – Nitrogen is a part of the RING Identify these examples: NH2 CH3 CH3 NH N CH3 1⁰ Aromatic Amine Aniline 2⁰ Aromatic Amine 3⁰ Aliphatic Amine Amphetamines • 3 examples Amphetamine Methamphetamine Phentermine • Common physiological effect: – Reduce fatigue and diminish hunger by raising glucose levels • Prescribed to: – Counter mild depression – Reduce hyperactivity in children – Suppress appetites NH2 Amphetamine CH3 CH3 NH Methamphetamine CH3 NH2 CH3 H3C Phentermine found in “Fen-Phen” • Illegally used to: – Reduce Fatigue – Elevate moods – mood enhancement • ADDICITIVE: Concentrated in Brain and CNS leads to sleeplessness, weight loss, & paranoia • Why? How does it work? – Action similar to Epinepherine – Excreted by adrenal gland – Makes glucose more available – Immediate glucose action – Commonly called “Adrenalin” HO HO OH NH CH3 Heterocyclic Amines • Heterocyclic Aliphatic Amines pyrrolidine piperidine N H N H • Heterocyclic Aromatic Amines pyrrol pyridine N H N 1. How many hydrogens does piperidine have? – 11 C5H11N 2. How many hydrogens does pyridine have? – 5 C5H5N 3. Write the molecular formula of each. 4. How many hydrogens does pyrrolidene have? – 9 C4H9N 5. How many hydrogens does pyrole have? – 5 C4H5N 6. Write the molecular formula of each. Alkaloids • Naturally occurring amine compounds – Found in roots, bark or fruits – Nitrogen found in ring – “Alkaloid” because compounds are BASIC • Coniine – Water Hemlock – Causes weakness, labored respiration, paralysis and death • Nicotine – Tobacco plant – Addictive in small doses – Large doses cause depression, nausea, vomiting • Cocaine – Leaves of Coca plant – CNS stimulant H H N N H CH3 N (s) Coniine CH3 (s) Nicotine H H H H H H O H H H H O O N H H H O H H H H H H H H Cocaine Section 16.3 Nomenclature • Same naming system as ALCOHOLS • Drop the “ending” or “e” of the parent chain and add “amine” H3C NH2 H3C NH2 NH2 CH3 H2N 1,6-hexandiamine 2-propanamine 1-phenyl-1-ethanamine Write the name or formula 1. CH3(CH2)5NH2 1-hexanamine H3C 2. 1-phenyl-2-propanamine NH2 3. Cyclohexanamine NH2 4. 1,4-butandiamine NH2 H2N • IUPAC nomenclature retains the common name: NH2 NH2 Aniline H3C CH3 • __Toluidine___ is methyl substituted aniline – 2-methyl aniline = o-toluidine • Largest group bonded to nitrogen is the parent amine when nitrogen has 2 or more carbon groups bonded to it (IUPAC) • Groups attached to Nitrogen are numbered as “N” NH2 CH3 • N-methylaniline HN H3C • N,N-dimethyl cyclopentanamine N CH3 • N-ethyl-2-propanamine H3C CH3 HN • N-ethyl-N-methyl ethanamine CH3 CH3 N H3C CH3 Common Names • Common names for aliphatic amines list the groups bonded to the nitrogen in alphabetical CH order. H 3C 3 H3C H3C NH2 Methylamine C CH2 NH2 N H 2C CH3 tert-butylamine • Draw the structure of Cyclohexylmethylamine Isopropylamine Triethylamine CH3 CH3 diethylmethylamine Amine Salts • When 4 atoms or groups are bonded to the nitrogen • Named as a salt with an “anion” (chloride) • Ending “amine” is replaced with -ammonium_ • Ending “aniline” is replaced with -anilinium • Ending “pyridine” is replaced with -pyridinium • Ammonium chloride H H Cl + N - H H • Tetramethyl ammonium chloride H C 3 CH3 + N CH3 • Pyridinum chloride • Anilinium chloride + H + Cl N H H Cl N H - - Cl CH3 - Section 16.4 Physical Properties • Amines are ___POLAR__ molecules • Primary and Secondary amines will form ____H-Bonds___ between themselves • Tertiary amines will NOT form __H-Bonds____ between themselves • ALL amines will form _____H-Bonds___ with WATER! • The N-H h-bond is WEAKER than the O-H hbond. • Methanamine vs. Methanol CH3NH2 CH3OH Molar Mass 31.1 32.0 Boiling Point -6.3⁰C 65.0⁰C • Why? •Stronger H-Bonding between Methanol Molecules Section 16.5 Basicity • Amines are _Slightly Strong to Weak Bases__ • Example: H H3C N H + H O H + H3C N H H H • Base dissociation constants [CH 3 NH 3 ][OH ] 4 Kb 4.37 x10 [CH 3 NH 2 ] + - HO • 2 ways to measure “base” strength – pKb -Log of Kb • Smaller values = Stronger Bases <3 = Very Strong base >6 = Weak Base – pH • Larger values = Stronger Bases 13-14 = Very Strong base 8-10 = Weak Base Amine pKB Base Nature Aliphatic 3.0-4.0 Slightly Strong Ammonia 4.74 Aromatic 8.5-9.5 Weak For each Set, choose which is the more “basic” NH2 NH2 CH3 N N H NH2 H3C NH3 • Basicity and Blood – Most Aliphatic amines are fairly strong bases – How will they be found in the blood stream? • In their “Ionic” or “Ammonium” Form • Example: Dopamine HO HO NH2 HO HO H+ NH H HO Section 16.6 REACTIONS • Amines react with strong acids to form watersoluble salts HO HO H NH2 + HO HO H H H + N H H Cl + HO (R) Norepinephrine Only Slightly water soluble (R) Norepinephrine hydrochloride Very water soluble Cl - Solubility of amine type DRUGS • Many drugs have “∙HCl” in their chemical formula • WHY? – High molecular weight amines are insoluble in blood – Treated with an acid – they form a water/blood soluble salt – Amines are also susceptible to oxidation from atmospheric O2. – Amine salts are less likely to be oxidized and retain a longer shelf life – LOOK AT LABELS! • Examples – Phenylephrine∙HCl H H HO N HO CH3 HCl • Is it (R) or (S)? – (Neo-Synephrine) – Procaine∙HCl – (Novocain) CH3 O N O CH3 HCl H2N O CH3 – Methadone ∙HCl – (Methadone) N CH3 HO – Albuterol ∙HCl – (Proventil) CH3 HCl CH3 H N CH3 HO CH3 HCl CH3 HO Chapter 17: Aldehydes & Ketones Naming Introduction Aldehydes • Terminal –CHO group • Suffix: “al” • Naming similar to alcohols O H3C CH3 C C H3C Ketones • Internal –C=O group • Suffix: “one” • Naming similar to alcohols H O Name the Following CH3 O O H3C CH3 CH3 CH3 H3C H H3C CH3 O CH3 Cl O Draw the Following • 1,6-dichloro-3-hexanone • 5-methylheptanal • 1,6-hexandial • 3-bromo-2-butanone Sections 17.1-17.3 • Order of Precedence of Main Functional Groups Class Group Suffix if highest Prefix if Lower Carboxylic acid Highest -COOH -oic acid Never Lower! Aldehyde -CHO -al oxo Ketone -C=O -one oxo Alcohol -OH -ol hydroxy Amine -NH2 -amine amino • Examples: Draw the following – 2-aminohexanal – 1-amino-2-hydroxyl-3-pentanone – 5-hydroxyl-2-hexenal – 3-methyl-2-oxobutanal – 4-amino-3-penten-2-one • Name the following H3C O O CH2 H3C CH3 OH O OH O CH3 O OH NH2