amine
... Aryl amines are generally less basic than alkylamines • Nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring p electron system and less available for bonding to H+ ...
... Aryl amines are generally less basic than alkylamines • Nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring p electron system and less available for bonding to H+ ...
Preparation and reactions of some lower tungsten halides and
... The following transformations have been observed (16). solid o (152° - 226.93°) : log solid B (226.93°- 284°) : log liquid (284° - 362°) : log ...
... The following transformations have been observed (16). solid o (152° - 226.93°) : log solid B (226.93°- 284°) : log liquid (284° - 362°) : log ...
synthetic approaches for quinoline and isoquinoline
... heterocylic compounds, such as pyridine derivatives, quinoline is often reported as an environmental contaminant associated with facilities processing oil shale or coal, and has also been found at legacy wood treatment sites.Owing to high water quinoline ha ...
... heterocylic compounds, such as pyridine derivatives, quinoline is often reported as an environmental contaminant associated with facilities processing oil shale or coal, and has also been found at legacy wood treatment sites.Owing to high water quinoline ha ...
Chapter 19
... Create quaternary nitrogen – nitrogen is prevented to invert with four covalent bonds ...
... Create quaternary nitrogen – nitrogen is prevented to invert with four covalent bonds ...
Lectures 4-6
... Collins Oxidation (CrO3 • 2pyridine) TL 1969, 336 - CrO3 (anhydrous) + pyridine (anhydrous) ...
... Collins Oxidation (CrO3 • 2pyridine) TL 1969, 336 - CrO3 (anhydrous) + pyridine (anhydrous) ...
cleavage of methyl ethers with iodotrimethylsilane
... as a result of hydrolysis by contact with moisture. The amount of by-products, including cyclohexyl iodide, is reduced by the presence of pyridine. Hindered pyridine bases such as 2,6-di-tert-butyl-4methylpyridine5 have also been used for this purpose, by the submitters. The pyridine bases do not ap ...
... as a result of hydrolysis by contact with moisture. The amount of by-products, including cyclohexyl iodide, is reduced by the presence of pyridine. Hindered pyridine bases such as 2,6-di-tert-butyl-4methylpyridine5 have also been used for this purpose, by the submitters. The pyridine bases do not ap ...
Novel Synthesis of Schiff bases Bearing Glucosamine Moiety
... In conclusion, the procedure has been used for conversion of DGlucosamine into the corresponding Schiff base molecules through protection of its hydroxyl groups, followed by condensation with substituted aldehydes. Handling, safety, mild reaction conditions, good to excellent yields of the products ...
... In conclusion, the procedure has been used for conversion of DGlucosamine into the corresponding Schiff base molecules through protection of its hydroxyl groups, followed by condensation with substituted aldehydes. Handling, safety, mild reaction conditions, good to excellent yields of the products ...
NICKEL(II) PINCER COMPLEXES SUPPORTED BY 2,6
... class of dianionic, tridentate, nitrogen-based ligands in coordination chemistry, was prepared starting with a modified method for the synthesis of pyrrolyl pyridine. This modified procedure is simpler, less time consuming making it cheaper than the classical method and provides 2,6-bis(3,5-ditolyl- ...
... class of dianionic, tridentate, nitrogen-based ligands in coordination chemistry, was prepared starting with a modified method for the synthesis of pyrrolyl pyridine. This modified procedure is simpler, less time consuming making it cheaper than the classical method and provides 2,6-bis(3,5-ditolyl- ...
Reactions hydroxyl groups part-I
... Remember anomeric center acts as an alcohol or aldehyde Describe the three types of hydroxyl groups found in sugars Realize the importance of protec,on to allow regioselec,ve rxns of those unprotected Ex ...
... Remember anomeric center acts as an alcohol or aldehyde Describe the three types of hydroxyl groups found in sugars Realize the importance of protec,on to allow regioselec,ve rxns of those unprotected Ex ...
Organic Chemistry - City University of New York
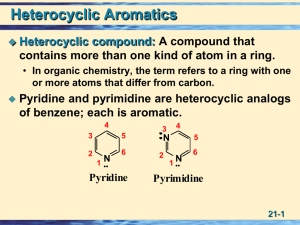
... compound: A compound that contains more than one kind of atom in a ring. • In organic chemistry, the term refers to a ring with one or more atoms that differ from carbon. ...
... compound: A compound that contains more than one kind of atom in a ring. • In organic chemistry, the term refers to a ring with one or more atoms that differ from carbon. ...
Chapter 10 - UCSB CLAS
... IV. Arene Oxides ⇒ In this chapter you will see arene oxides undergoing either nucleophilic substitution reactions same as epoxides in basic/neutral conditions or rearrangement reactions to produce phenol (primary focus) – since there’s a carbocation formed in the rearrangement reaction this will de ...
... IV. Arene Oxides ⇒ In this chapter you will see arene oxides undergoing either nucleophilic substitution reactions same as epoxides in basic/neutral conditions or rearrangement reactions to produce phenol (primary focus) – since there’s a carbocation formed in the rearrangement reaction this will de ...
HMDS+TMCS+Pyridine - Sigma
... Nonpolar organic solvents such as hexane, ether, benzene, and toluene are excellent solvents for the reagent and the reaction products; they do not accelerate the rate of reaction. Polar solvents such as pyridine, dimethylformamide (DMF), dimethylsulfoxide (DMSO), tetrahydrofuran (THF), and acetonit ...
... Nonpolar organic solvents such as hexane, ether, benzene, and toluene are excellent solvents for the reagent and the reaction products; they do not accelerate the rate of reaction. Polar solvents such as pyridine, dimethylformamide (DMF), dimethylsulfoxide (DMSO), tetrahydrofuran (THF), and acetonit ...
Tr-dT, 2-cyanoethanol
... 40 min. and ' P WMR spectrum was recorded to check completion of the reaction. The mixture was diluted by 54 ml of water (t = 1°C) and immediatly applied to a column (25 x 170 mm) with Molselect-DEAE 25 ion-exchange resin in HC07 form with the flow rate 70 ml/h. A glass filter was used to remove DCC ...
... 40 min. and ' P WMR spectrum was recorded to check completion of the reaction. The mixture was diluted by 54 ml of water (t = 1°C) and immediatly applied to a column (25 x 170 mm) with Molselect-DEAE 25 ion-exchange resin in HC07 form with the flow rate 70 ml/h. A glass filter was used to remove DCC ...
Organic Chemistry - Snow College | It's SNOWing
... (2.5) and H (2.1) is 0.4. • This creates a bond with low polarity • show little association by hydrogen bonding • have lower boiling points and are less soluble in water than alcohols of comparable MW ...
... (2.5) and H (2.1) is 0.4. • This creates a bond with low polarity • show little association by hydrogen bonding • have lower boiling points and are less soluble in water than alcohols of comparable MW ...
Solvothermal Synthesis of Polyazomethine Microspheres
... g), and glacial acetic acid (150mL) were refluxed for 6 h. After cooled to room temperature, the mixture was separated by filtered and washed first with acetic acid (50%) and then with cold ethanol. The crude product was directed used without further treatment. To the solution of the obtained nitro ...
... g), and glacial acetic acid (150mL) were refluxed for 6 h. After cooled to room temperature, the mixture was separated by filtered and washed first with acetic acid (50%) and then with cold ethanol. The crude product was directed used without further treatment. To the solution of the obtained nitro ...
Lecture
... Pyridine is an analog of benzene in which one of the СН units is replaced by nitrogen. The nitrogen lone pair is located in an sp2 hybrid orbital which is perpendicular to the system of the ring. Various values have been deduced for the empirical resonance energy of pyridine, but it would appear t ...
... Pyridine is an analog of benzene in which one of the СН units is replaced by nitrogen. The nitrogen lone pair is located in an sp2 hybrid orbital which is perpendicular to the system of the ring. Various values have been deduced for the empirical resonance energy of pyridine, but it would appear t ...
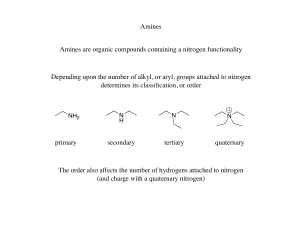
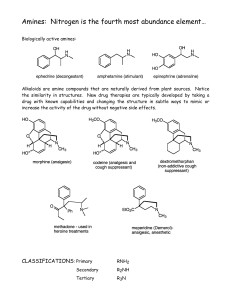
Amines: Nitrogen is the fourth most abundance element…
... Compounds are highly polar resulting in higher boiling points than alkanes or alkenes. Amines are also capable of hydrogen bonding, which increases the boiling points. Nitrogen is less electronegative than oxygen. The bond is not as strong and the boiling point is therefore lower than that of a simi ...
... Compounds are highly polar resulting in higher boiling points than alkanes or alkenes. Amines are also capable of hydrogen bonding, which increases the boiling points. Nitrogen is less electronegative than oxygen. The bond is not as strong and the boiling point is therefore lower than that of a simi ...
$doc.title
... • A heterocycle is a cyclic compound that contains atoms of two or more elements in its ring, usually C along with N, O, or S ...
... • A heterocycle is a cyclic compound that contains atoms of two or more elements in its ring, usually C along with N, O, or S ...
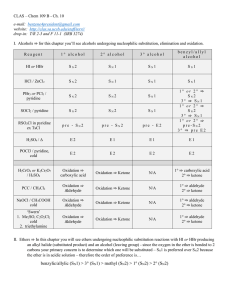
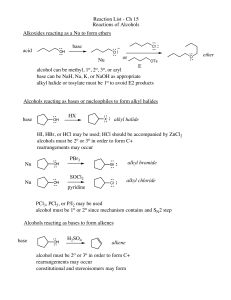
Conversion of Alcohols into Alkyl Halides
... 1o and 2o Alcohols: best to use SOCl2, PBr3, or P/I2 All are SN2 Reactions SOCl2 pyridine OH ...
... 1o and 2o Alcohols: best to use SOCl2, PBr3, or P/I2 All are SN2 Reactions SOCl2 pyridine OH ...
Alcohols/Wade: Reactions
... 10. Compound A is an optically active alcohol. Treatment with chromic acid converts A to a ketone B. In a separate reaction A is treated with PBr3, converting A to C. Compound D is purified and reacted with magnesium and ether. Compound B is added to the resulting solution of the Grignard reagent. ...
... 10. Compound A is an optically active alcohol. Treatment with chromic acid converts A to a ketone B. In a separate reaction A is treated with PBr3, converting A to C. Compound D is purified and reacted with magnesium and ether. Compound B is added to the resulting solution of the Grignard reagent. ...
Aromatic heterocycles 1: structures and reactions
... probably about two-thirds of—organic compounds belong to this class, and they number among them some of the most significant compounds for human beings. If we think only of drugs we can define the history of medicine by heterocycles. Even in the sixteenth century quinine was used to prevent and trea ...
... probably about two-thirds of—organic compounds belong to this class, and they number among them some of the most significant compounds for human beings. If we think only of drugs we can define the history of medicine by heterocycles. Even in the sixteenth century quinine was used to prevent and trea ...
Reaction List - Ch 15 Reactions of Alcohols Alkoxides reacting as a
... Alkoxides reacting as a Nu to form ethers base ...
... Alkoxides reacting as a Nu to form ethers base ...
Electrochemical Investigations of W(CO) (L) and W(CO) (L) Complexes:
... • The bulk oxidation peak potential shifts to lower potentials • A reversible redox couple assigned to [W(CO)4(py)2]0/+ emerges (see Figure 2).2 The results shown in Figure 1 indicate that the seventeen electron radical cation undergoes a rapid ligand exchange in which a carbon monoxide is replaced ...
... • The bulk oxidation peak potential shifts to lower potentials • A reversible redox couple assigned to [W(CO)4(py)2]0/+ emerges (see Figure 2).2 The results shown in Figure 1 indicate that the seventeen electron radical cation undergoes a rapid ligand exchange in which a carbon monoxide is replaced ...
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH-) replaced by a nitrogen atom. The pyridine ring occurs in many important compounds, including azines and the vitamins niacin and pyridoxal.Pyridine was discovered in 1849 by the Scottish chemist Thomas Anderson as one of the constituents of bone oil. Two years later, Anderson isolated pure pyridine through fractional distillation of the oil. It is a colorless, highly flammable, weakly alkaline, water-soluble liquid with a distinctive, unpleasant fish-like odor.Pyridine is used as a precursor to agrochemicals and pharmaceuticals and is also an important solvent and reagent. Pyridine is added to ethanol to make it unsuitable for drinking (see denatured alcohol). It is used in the in vitro synthesis of DNA, in the synthesis of sulfapyridine (a drug against bacterial and viral infections), antihistaminic drugs tripelennamine and mepyramine, as well as water repellents, bactericides, and herbicides. Some chemical compounds, although not synthesized from pyridine, contain its ring structure. They include B vitamins niacin and pyridoxal, the anti-tuberculosis drug isoniazid, nicotine and other nitrogen-containing plant products. Historically, pyridine was produced from coal tar and as a by-product of the coal gasification. However, increased demand for pyridine resulted in the development of more economical methods of synthesis from acetaldehyde and ammonia, and more than 20,000 tonnes per year are manufactured worldwide.