* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Reactions hydroxyl groups part-I
Survey
Document related concepts
Transcript
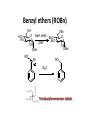
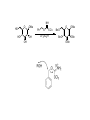
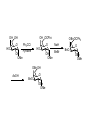
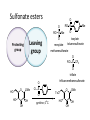
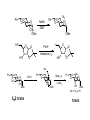
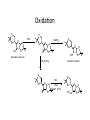
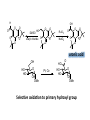
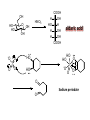
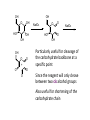
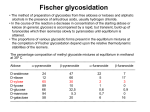
LECTURE 5 REACTIONS HYDROXYL GROUPS PART I NOTHING NEW OR MYSTERIOUS The complica,ons arise only because of there are so many of them!! As nucleophiles, analog to non-‐sugar alcohols The majority of the chemistry is related to the forma,on of esters and ethers. Carboxylate esters Acetates –O(CO)CH3 or –OAc Benzoates –O(CO)C6H5 or –OBz Sulfonate esters Tosylate –O(SO2) C6H4CH3 or –OTs Mesylate –O(SO2) CH3 or –OMs Triflate –O(SO2) CF3 or –OTf Ethers Benzyl –OCH2C6H5 or –OBn Trityl –OC(C6H5)3 or –OTr Trimethylsilyl –OSi(CH3)3 or –OTMS Tert-‐butyldimethylsilyl –OSi(CH3)2(C(CH3)3) or –OTBS or –OSiMe2But or -‐OTBDMS Tert-‐butyldiphenylsilyl –OSi(C6H5)2(C(CH3)3) or –OTBS or –OSiPh2But or -‐OTBDPS HO HO OH O OH OH primary OH-6 HO HO OH O OH OH secondary OH-2,3,4 HO HO OH O OH OH anomeric OH-1 The functionality at the anomeric center can react in two ways as an alcohol or as an aldehyde act as aldehyde to form acetal Effec,vely protect the anomeric center and prevents further reac,vity Equilibrium of α- and β-hydroxyls at the anomeric position Potential competing inter-conversion between α and β forms inter-conversion between pyranose and furanose forms Solubility: very polar in nature very soluble in polar solvents, especially with possible H-bonding Insoluble in non-polar solvents for organic reactions, pdt purification and manipulation Selective protection of particular hydroxyl groups allows the regioselctive rxn of those unprotected Common starting point: protect the majority or even all of the free -OH AcetylaKon Ester Acetals Ether Nucleophilic SubsKtuKon oxidaKon ReducKon AcetylaKon Acetals Ether Ester Nucleophilic SubsKtuKon oxidaKon ReducKon Esters: non-‐nucleophilic and stable to a wide range of Condi,ons Frequently used protect groups Less polar than the corresponding alcohols Acetyla,on Most commonly used esters SKrring the alcohol with aceKc anhydride and a base Base mop up the aceKc acid generated, and catalyses the rxn itself Nucleophilic catalysis and base Uncatalysed rxn is very slow at rt Lewis acid catalysis But with possible compeKng process of mutarotaKon 1. Acetyla,on with ace,c anhydride/ pyridine propor,ons as solvents Equal HO HO OH O OH Ac2O, pyridine fast OH Sugars in a boYle is a mixture of α and β. /(py/fu) HO HO AcO AcO OAc OAc slow OH O OAc O + Ac2O, pyridine OH OH Ra,o depends on par,cular sugars fast AcO AcO OAc O OAc OAc 2. Acetyla,on with ace,c anhydride/ sodium acetate Rela,vely weak base, sluggish rxn at rt Carry out at 100°C, usually generate β-‐acetates Mutarota,on is much faster than the acetyla,on HO HO OH O OH OH fast HO HO OH O OH OH Ac2O, NaOAc 100oC slow AcO AcO OAc O OAc β Hydroxyl is more nucleophilic than the axial α οne OAc β-‐ occupies a less hindered equatorial than the axial of α- repulsive effect between the ring oxygen lone pair orbitals and the lone pair orbitals on the β-‐ anomeric oxygen atom KineKc anomeric effect 3. Acetyla,on with ace,c anhydride/ Lewis acid catalysis Strong Lewis acid catalyses the equilibrium of α-‐ and β-‐, a^er catalyses the rxn Thus equilibrium of α-‐ and β-‐acetates will occur a^er the rxn Anomeric effect leads us α-‐acetates Protec,ng groups Sa,sfy several important criteria 1) They should be formed in good yield, 2) they should be stable to subsequent rxn condi,ons, 3) They should be readily removed under appropriate condi,ons AcetylaKon Acetals Ether Ester Nucleophilic SubsKtuKon oxidaKon ReducKon AcetylaKon Acetals Ether Ester Nucleophilic SubsKtuKon oxidaKon ReducKon Benzyl ethers (ROBn) HO HO OH O NaH, BnBr OH OMe RO- DMF BnO BnO OBn O OBn OMe RO Br SN2 Tetrabutylammonium iodide NH HO O HO OMe OH OH Ph O CCl3 CF3SO3H BnO O BnO OMe OBn OBn Cleavage Cataly,c hydrogena,on Heterogeneous catalyst Palladium on carbon (Pd/C) When the metal is distributed over finely-divided carbon, the surface area is larger and the catalyst is more reactive. Palladium hydroxide (Pearlman’s catalyst) For the removal of sterically inaccessible/mul,ple benzyl groups OR MeO para-methoxybenzyl (PMB) Single electron oxidising reagent (NH4)2Ce(NO3)6 Ceric ammonium nitrate (CAN) O Cl CN Cl CN O DDQ 2,3-‐Dichloro-‐5,6-‐Dicyanobenzoquinone Trityl ethers Triphenylmethyl ether Very selec,ve for the primary hydroxyl group Forma,on via an SN1 type process By treatment of unprotected sugar etc. etc. Resonance stabilisation of the trityl cation OH OCPh3 OH OH HO O OH OMe Ph3CCl HO Pyridine OH OMe OBn OH AcOH O BnO O OBn OMe OBn OCPh3 NaH BnBr BnO O OBn OMe Silyl ethers R OH R' Si R'' Cl R''' -H+ R' R O Si Cl R'' R''' 'ate' complex Ph But Si N But N H Imidazole acts as a nucleophilic catalyst and forms intermediates, e.g. R O R' Si R'' R''' Trimethylsilyl (TMS) HO HO OH OH O Me3SiCl Pyridine Me3SiO Me3SiO OSiMe3 OSiMe3 O OMe Tert-‐Butyldimethylsilyl (TBDMS or TBS) OMe Si HO HO OH OH O ButPh2SiCl imidazole OMe HO HO O OH O OMe cleavage acid, may also cause removal of othe racid labile protec,ng groups Tetrabutylammonium fluoride (TBAF) Rela,ve rate of acid hydrolysis (stability increase in order) R-OSiMe3 R-OSiMe2But R-OSiPh2But 1 1E-3 1E-5 AcetylaKon Acetals Ether Ester Nucleophilic SubsKtuKon oxidaKon ReducKon Acetate, and R O Removed by nucleophile O = R---OBz Most commonly methoxide in trans-‐esterifica,on rxn benzoyl ester Potassium carbonate in methanol Sodium methoxide in methanol (zémplen deacetyla,on) In both cases, methoxide in cataly,c Milder condi,ons, such as treatment with primary amines, are also effec,ve for the removal of certain esters, and can do selec,ve deprotec,on rxn Sulfonate esters O RO S O O RO S Me Leaving group ProtecKng group O mesylate Me tosylate toluenesulfonate methanesulfonate O RO S CF3 O triflate trifluoromethanesuflonate O HO O OMe Cl S O HO OH OH pyridine, 0oC TsO O HO OMe OH OH AcetylaKon Acetals Ether Ester Nucleophilic SubsKtuKon oxidaKon ReducKon Nucleophilic subs,tu,on RXN Retarded by the presence of electron withdrawing oxygen which are β to the carbon atom at which the displacement is taking place SN2 not SN1 Electron withdrawing effect greatly destabilises any carbonium in in SN1 β-‐oxygen effect Nu LG HO HO O LG OMe Ph O O HO O NaN3 OTs OMe DMF O HO Ph N3 O OMe I Ph3P O Imidazole, I2 O O O O O HO O NaN3 or Ph NuPh O O TsO O OH OMe SN2 trans KOH Ph O O O O OMe LiAlH4 O O Nu O OH OMe Nu = N3 or H trans AcetylaKon Acetals Ether Ester Nucleophilic SubsKtuKon oxidaKon ReducKon Oxida,on O O PCC O O HO O NaBH4 O O O O O O O O O HO diacetone glucose O diacetone allose Ph3P=CH2 O O O O BH3 O O O then NaOH, H2O2 O HO O O O PCC Oxida,on Pfitzner–Moffa` oxidaKon Swern oxidaKon H OH O O O O O DMSO HO O oxalyl chloride O O O O O O O RuCl3 O NaIO4 O O O uronic acid OH HO HO O OH OMe HO Pt, O2 HO HO O O OH OMe SelecKve oxidaKon to primary hydroxyl group OH HO HO -O O I O OH HNO3 OH O HO O HO H HO H H COOH OH H OH OH COOH aldaric acid HO O HO I -O O O O O Sodium periodate OH OH O HO OH OH OH O + O O O HO O NaIO4 O OH OH O O NaIO4 Par,cularly useful for cleavage of the carbohydrate backbone at a specific point Since the reagent will only cleave between two cis alcohol groups Also useful for shortening of the carbohydrate chain AcetylaKon Acetals Ether Ester Nucleophilic SubsKtuKon oxidaKon ReducKon Reduc,on to prepare deoxy sugars O O O O O HO O O CS2, NaOH O then MeI S MeS O O O Bu3SnH O O AIBN O O Xanthate ester Barton-‐McCombie deoxygenaKon Bu3Sn Bu3Sn S R O SMe R O S O R H SnBu3 SMe RH + Bu3Sn product chain carrier regenerated Other possible ways Nucleophilic displacement of leaving groups, e.g. OMs or OTs, with hydride from reducing agents such as LiAlH4 is not generally a good route Nucleophilic subs,tu,on is much slower in carbohydrates due to the β-‐oxygen effect Under basic condi,ons, side reac,ons such as elimina,on rxns occur preferen,ally with reducing agents Hydrogenolysis of halides in the presence of mild base is prefered. summary Describe the three types of hydroxyl groups found in sugars Remember anomeric center acts as an alcohol or aldehyde Describe the three types of hydroxyl groups found in sugars Realize the importance of protec,on to allow regioselec,ve rxns of those unprotected Explain the pdts of acetyla,ons under different condi,ons Know how to selec,vely protect the primary hydroxyl group Recall the method in forming and cleaving ethers and esters