Neurotransmitters - Mayfield City Schools
... • Involved in attention and arousal in CNS • In PNS involved in fight or flight • As a neurotransmitter works to increase heart rate ...
... • Involved in attention and arousal in CNS • In PNS involved in fight or flight • As a neurotransmitter works to increase heart rate ...
Exam 1 Solution Key
... The resonance structures illustrate the fact that there is extensive electron delocalization within the conjugate base, which makes the conjugate base exceptionally stable and a “weak base”; therefore, the undissociated acid would have considerable tendency to dissociate, making it a strong acid. ...
... The resonance structures illustrate the fact that there is extensive electron delocalization within the conjugate base, which makes the conjugate base exceptionally stable and a “weak base”; therefore, the undissociated acid would have considerable tendency to dissociate, making it a strong acid. ...
Dess-Martin Oxidation
... Recyclable 2nd generation ionic liquids as green solvents for the oxidation of alcohols with hypervalent iodine reagents J. S. Yadav, B. V. S. Reddy, A. K. Basak, A. V. Narsaiah, Tetrahedron, ...
... Recyclable 2nd generation ionic liquids as green solvents for the oxidation of alcohols with hypervalent iodine reagents J. S. Yadav, B. V. S. Reddy, A. K. Basak, A. V. Narsaiah, Tetrahedron, ...
Chem 400 Review Chem 350 JJ.S17
... Alcohols are names with the suffix –ol The acidity of an alcohol/phenol can be assessed using its conjugate base: Consider Atomic size/Electronegativity, Resonance, Induction, Orbital Type (ARIO) Electron withdrawing substituents stabilize conjugate bases while electron donating ones destabili ...
... Alcohols are names with the suffix –ol The acidity of an alcohol/phenol can be assessed using its conjugate base: Consider Atomic size/Electronegativity, Resonance, Induction, Orbital Type (ARIO) Electron withdrawing substituents stabilize conjugate bases while electron donating ones destabili ...
No Slide Title
... Amines are also nucleophiles (lone pair on N) and can attack halogenoalkanes to produce a 2° amine. This too is a nucleophile and can react further producing a 3° amine and, eventually an ionic quarternary ammonium salt. C2H5NH2 + C2H5Br ...
... Amines are also nucleophiles (lone pair on N) and can attack halogenoalkanes to produce a 2° amine. This too is a nucleophile and can react further producing a 3° amine and, eventually an ionic quarternary ammonium salt. C2H5NH2 + C2H5Br ...
aminepp - Knockhardy
... Amines are also nucleophiles (lone pair on N) and can attack halogenoalkanes to produce a 2° amine. This too is a nucleophile and can react further producing a 3° amine and, eventually an ionic quarternary ammonium salt. C2H5NH2 + C2H5Br ...
... Amines are also nucleophiles (lone pair on N) and can attack halogenoalkanes to produce a 2° amine. This too is a nucleophile and can react further producing a 3° amine and, eventually an ionic quarternary ammonium salt. C2H5NH2 + C2H5Br ...
+ H 2 O(g)
... First, the oxidation number of each atom have to be calculated and check if any of them changed. None of the oxidation numbers changes. CaO is a basic oxide as well as NH4+ is the protonated ammonia, then it can deliver H+ as an acid, then this reaction might be an acid base reaction. It occurs in h ...
... First, the oxidation number of each atom have to be calculated and check if any of them changed. None of the oxidation numbers changes. CaO is a basic oxide as well as NH4+ is the protonated ammonia, then it can deliver H+ as an acid, then this reaction might be an acid base reaction. It occurs in h ...
PAKISTAN SHIPOWNERS` GOVERNMENT COLLEGE,
... * Homologous Series * Octane number *Acid value (ii) a) Define Isomerism. Explain the types of Isomerism with examples. b) Explain why Alkanes are less reactive than Alkenes? (iii) a) Write a note on polymerization and its types. b) Distinguish by simple chemical test, *Aldehyde and Ketone * Ethene ...
... * Homologous Series * Octane number *Acid value (ii) a) Define Isomerism. Explain the types of Isomerism with examples. b) Explain why Alkanes are less reactive than Alkenes? (iii) a) Write a note on polymerization and its types. b) Distinguish by simple chemical test, *Aldehyde and Ketone * Ethene ...
chemistry important question i
... (b) Draw the structures of the following : (i) H4P2O7 (Pyrophosphoric acid) (ii) XeF2 8.(a) Draw the structures of the following : (i) XeF4 (ii) H2S2O7 (b) Account for the following : (i) Iron on reaction with HCl forms FeCl2 and not FeCl3. (ii) HClO4 is a stronger acid than HClO. (iii) BiH3 is the ...
... (b) Draw the structures of the following : (i) H4P2O7 (Pyrophosphoric acid) (ii) XeF2 8.(a) Draw the structures of the following : (i) XeF4 (ii) H2S2O7 (b) Account for the following : (i) Iron on reaction with HCl forms FeCl2 and not FeCl3. (ii) HClO4 is a stronger acid than HClO. (iii) BiH3 is the ...
carbohydrates
... of the ring atoms is 5 or 6. As pyrane is a six-membered ring containing one oxygen, while furane is similar with a five-membered ring, the cyclohemiacetale or cyclohemiketale sugar rings are called as „pyranose” (6-membered) or „furanose” (5-membered) structures. Pyranose is characteristic of aldoh ...
... of the ring atoms is 5 or 6. As pyrane is a six-membered ring containing one oxygen, while furane is similar with a five-membered ring, the cyclohemiacetale or cyclohemiketale sugar rings are called as „pyranose” (6-membered) or „furanose” (5-membered) structures. Pyranose is characteristic of aldoh ...
Organic Objectives
... state the # of atoms and # of elements in a molecule. draw isomers of molecules and recognize isomers. write the formula of a hydrocarbon, given its name and vice versa. give household examples of hydrocarbons (methane, ethane, propane, butane, octane). identify the “parent chain” looking ...
... state the # of atoms and # of elements in a molecule. draw isomers of molecules and recognize isomers. write the formula of a hydrocarbon, given its name and vice versa. give household examples of hydrocarbons (methane, ethane, propane, butane, octane). identify the “parent chain” looking ...
( i ) in enantioselective nhk reaction
... THF proved to be the best solvent for these catalytic asymmetric reactions and catalyst concentration of 0.025 M gave the best results In contrast to salen ligand 1, ligand 2 was able to effect an enantioselective addition of allyl iodide ...
... THF proved to be the best solvent for these catalytic asymmetric reactions and catalyst concentration of 0.025 M gave the best results In contrast to salen ligand 1, ligand 2 was able to effect an enantioselective addition of allyl iodide ...
AlCl3 in modern chemistry of polyfluoroarenes
... fluorine atoms in one aromatic ring and the transformations are systematized according to the type of the reactions taking place. 1. Friedel-Crafts alkylation and acylation reactions Among the reactions catalyzed by AlCl 3 , both in series of polyfluoroarenes and for nonfluorinated compounds the mos ...
... fluorine atoms in one aromatic ring and the transformations are systematized according to the type of the reactions taking place. 1. Friedel-Crafts alkylation and acylation reactions Among the reactions catalyzed by AlCl 3 , both in series of polyfluoroarenes and for nonfluorinated compounds the mos ...
Chemistry 1
... In a laboratory test, 27.3 g N2H4 and 51.3 g N2O4 are placed in a container to be reacted. a) Explain how the following calculations help to determine the limiting reactant for this reaction. Tell what the limiting reactant is. ...
... In a laboratory test, 27.3 g N2H4 and 51.3 g N2O4 are placed in a container to be reacted. a) Explain how the following calculations help to determine the limiting reactant for this reaction. Tell what the limiting reactant is. ...
No Slide Title
... • Secondary amines and carbonyls form Enamines – mechanism involves an immonium ion (also called iminium ion) lacking a hydrogen on the nitrogen atom ...
... • Secondary amines and carbonyls form Enamines – mechanism involves an immonium ion (also called iminium ion) lacking a hydrogen on the nitrogen atom ...
Biodiesel Session 2
... • Other side reactions may be: Reaction with bases in water or water to form free fatty acids and acylates O O ...
... • Other side reactions may be: Reaction with bases in water or water to form free fatty acids and acylates O O ...
11.Unit 10 Haloalkanes and Haloarenes.
... C-N bond. So RCN is major product. AgCN is covalent and so more electro negative N can attach to C and forms iso cyanides. Q4. Aryl halides cannot be prepared by the action of sodium halide on phenol in the presence H2SO4. Why? Ans. Due to resonance the carbon-oxygen bond in phenol has partial doubl ...
... C-N bond. So RCN is major product. AgCN is covalent and so more electro negative N can attach to C and forms iso cyanides. Q4. Aryl halides cannot be prepared by the action of sodium halide on phenol in the presence H2SO4. Why? Ans. Due to resonance the carbon-oxygen bond in phenol has partial doubl ...
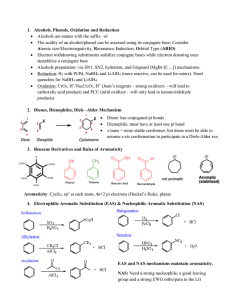
Oxidation of Alcohols
... 1. Acid catalyzed hydration 2. Oxymercuration 3. Hydroboration Hydrolysis of alkyl halides (Chapter 8) nucleophilic substitution Reaction of Grignard or organolithium reagents with ketones, aldehydes, and esters. (Chapter 14) ...
... 1. Acid catalyzed hydration 2. Oxymercuration 3. Hydroboration Hydrolysis of alkyl halides (Chapter 8) nucleophilic substitution Reaction of Grignard or organolithium reagents with ketones, aldehydes, and esters. (Chapter 14) ...
ppt
... Grignard Reagents. Conversion of an alkyl or aryl Grignard reagent to a carboxylic acid with an addition carbon (the CO2H group). The CO2H group is derived from CO2. Mg(0) R-Br ...
... Grignard Reagents. Conversion of an alkyl or aryl Grignard reagent to a carboxylic acid with an addition carbon (the CO2H group). The CO2H group is derived from CO2. Mg(0) R-Br ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.