* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download No Slide Title
Bottromycin wikipedia , lookup
Metal carbonyl wikipedia , lookup
Aldol reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff rearrangement wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Petasis reaction wikipedia , lookup
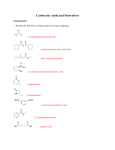
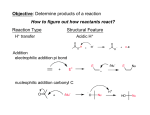
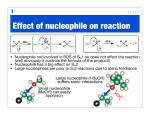
Chapter 12 Nucleophilic Addition and Substitution at Carbonyl Groups • • • • • • • • • Nucleophilic Addition to a Carbonyl Group Nucleophilic Addition of Hydrogen to Carbonyl groups Oxygen Nucleophiles Nitrogen Nucleophiles Nucleophilic Acyl Substitution of Carboxylic Acids and Derivatives Derivatives of Sulfonic and Phosphoric Acids Carbon Nucleophiles Synthetic Applications Spectroscopy Chem 3313 W.J. Baron Spring 1999 4MWF Chapter 12 Nucleophilic Addition and Substitution at Carbonyl Groups • Nucleophilic Addition to a Carbonyl Group – The Carbonyl Group • 3 Loci of Reactivity, Structure of C=O • Characteristics: bond length, hybridization, pKa, Dipole, Bond angle, resonance structures, molecular orbitals, physical properties, H-bonds – Possible Reactions of a Nucleophile with a Carbonyl Group • Nucleophilic Addition (with heteroatom or carbon nucleophile) • Nucleophilic Substitution (by heteroatom or carbon nucleophile) – Anions as Nucleophiles • Lewis base anions and neutral Lewis bases as Nucleophiles • Nucleophilicity – NH3 > H2O >HF (same row) – Iodide ion > Bromide > Chloride > Fluoride (same column) Chem 3313 W.J. Baron Spring 1999 4MWF Chapter 12 Nucleophilic Addition and Substitution at Carbonyl Group • Nucleophilic Addition of Hydrogen to Carbonyl groups – Complex Metal Hydride Reductions • • • • • • LiAlH4, LiBH4 and NaBH4 Aldehydes and Ketones reduced to primary and secondary R-OH Esters reduced to R-CH2-OH pri-, sec-, tert-Amides reduced to pri-, sec-, tert-amine Thiol Ester reduced to mercaptan Carboxylic Acid to primary Alcohol – Relative Reactivity of Carbonyl Compounds toward Hydride Reducing Agents • Aldehyde > Ketone > Thiol ester > Ester > Amide > Carboxylate • use NaBH4 with Aldehyde and Ketone, LiAlH4 on all above Chem 3313 W.J. Baron Spring 1999 4MWF Chapter 12 Nucleophilic Addition and Substitution at Carbonyl Groups • Oxygen Nucleophiles – Addition of Water; Hydrate Formation • • • • Base Catalyzed (2 steps: addition, followed by protonation) Acid Catalyzed (3 steps: protonation, addition, deprotonation) Generalized for oxygen and nitrogen nucleophiles Equilibrium of carbonyls and hydrates – Addition of Hydroxide Ion: The Cannizzaro Reaction and Hydride Transfer • Aromatic aldehydes and aldehydes lacking alpha hydrogen – Addition of Alcohols • Aldehydes form hemiacetals and acetals • Ketones form hemiketals and ketals • Acetals and Ketals as protecting groups Chem 3313 W.J. Baron Spring 1999 4MWF Chapter 12 Nucleophilic Addition and Substitution at Carbonyl Groups • Nitrogen Nucleophiles – Amines • Primary amines and carbonyls form Imines (also called Schiff base) – examples include, hydrazones, phenylhydrazones, 2,4dinitrophenylhydrazones, semicarbazones, tosylhydrazones, oximes • Secondary amines and carbonyls form Enamines – mechanism involves an immonium ion (also called iminium ion) lacking a hydrogen on the nitrogen atom – Other Nitrogen Nucleophiles • Formation of derivatives shown under primary amines above – used for identification of ketone or aldehyde Chem 3313 W.J. Baron Spring 1999 4MWF Chapter 12 Nucleophilic Addition and Substitution at Carbonyl Groups • Nucleophilic Acyl Substitution of Carboxylic Acids and Derivatives – Relative Stability of Carboxylic Acid Derivatives • General RCO-X converted to RCO-Y • Reactivity acid chloride > thiol ester = anhydride > ester > carboxylic acid > amide > carboxylate anion – Interconverison of Carboxylic Acid Derivatives • • • • Thiol Esters acid catalyzed hydrolysis to Acid (by Base to RCO2 ion) Ester acid catalyzed hydrolysis to Acid (reverse reaction in ROH) Transesterification Amides hydrolyzed to Acid – Carboxylic Acid Chlorides • Preparation from thionyl chloride • with H2O forms acid, with ROH forms ester, with amine forms amide, with LiAlH4 forms alcohol – Reactions of Acid Anhydrides – Hydrolysis of Nitriles to Carboxylic Acids Chem 3313 W.J. Baron Spring 1999 4MWF Chapter 12 Nucleophilic Addition and Substitution at Carbonyl Groups • Derivatives of Sulfonic and Phosphoric Acids – Sulfonic Acid Derivatives • • • • • Formation of sulfonyl chloride (RSO2Cl) Nucleophilic substitution with alcohols to form tosylates Nucleophilic SN2 displacement of tosylates Relative Rates of Displacement of various Leaving Groups Nucleophilic substitution with amines to form sulfonamides – Phosphoric Acid Derivatives • Similar reactions as sulfonic acid derivatives Chem 3313 W.J. Baron Spring 1999 4MWF Chapter 12 Nucleophilic Addition and Substitution at Carbonyl Groups • Carbon Nucleophiles – Cyanide Ion • Cyanohydrin formation • Strecker synthesis to form -amino acids – Grignard Reagents • with Ketones to form 3 alcohols • with Esters to form 3 alcohols • with carbon dioxide to form carboxylic acid – Organolithium Reagents • with formaldehyde, aldehydes, ketones to 1 , 2 , 3 alcohol, respectively – The Wittig Reaction • ylide formation from Ph3P, alkyl halide and Base • ylide and carbonyl reaction to form alkenes Chem 3313 W.J. Baron Spring 1999 4MWF Chapter 12 Nucleophilic Addition and Substitution at Carbonyl Groups • Synthetic Applications – Review Table 12.2 p 643ff • Using Nucleophilic Additions and Substitutions (and Related Reactions) to Make Various Functional Groups • Spectroscopy • Review of Reactions • Summary Chem 3313 W.J. Baron Spring 1999 4MWF Chapter 12 Summary • Nucleophilic Substitution and Addition to C=O • Order of Reactivity towards Nucleophiles is aldehydes > ketones > esters > amides > carboxylate anions • Addition takes place if a poor leaving group is bonded to C=O, as in aldehydes and ketones • Substitution takes place if a good leaving group is bonded to C=O, as in acid chlorides, anhydrides, esters, amides and nitrides • Nucelophile reactivity C > N > O • Leaving group stability O > N > C Chem 3313 W.J. Baron Spring 1999 4MWF Chapter 12 Summary • Addition of water to C=O occurs rapidly, reversibly with acid or base catalysis • Addition of alcohols yields ketals or acetals • Nitrogen nucleophiles add to C=O to from imines from primary alcohols and enamines from secondary amines • Hydrazine or substitutes derivatives form hydrazones • Carboxylic acids derivatives interconverted by Nucleophilic acyl substitution • Reactivity acid chloride > thiol ester = anhydride > ester > carboxylic acid > amide > carboxylate anion • Carbon nucleophiles: nitriles, Grignard, organolithium and wittig reactions Chem 3313 W.J. Baron Spring 1999 4MWF